Food Safety Focus (138th Issue, January 2018) – Incident in Focus
Powdered Infant Formulas (PIF) are Not Sterile
– Salmonella is Not Acceptable
Reported by Dr. Ken CHONG, Scientific Officer,
Risk Assessment Section,
Centre for Food Safety
Background
In December 2017, the consumption of PIF was implicated as the vehicle of Salmonella outbreak among infants in France and the implicated products from concerned company had to be recalled. After confirming local availability, the Centre for Food Safety (CFS) instructed traders to recall concerned products. In this article, the presence of pathogens in PIF and relevant recommendations on safe preparation of PIF are discussed.
Pathogens of Concern in PIF
Food poisoning outbreaks associated with PIF raised international concerns years ago; the Food and Agriculture Organization (FAO) of the United Nations/World Health Organization (WHO) expert meetings evaluated specific microoganisms in PIF that resulted in cases of illness in infants, which concluded that PIF contaminated with Cronobacter species (formerly Enterobacter sakazakii) and Salmonella has been convincingly shown to be the vehicle and source of infection in infants. In fact, both Cronobacter species and Salmonella are able to survive in dry environments (like dried food and food processing environment) for prolonged periods of time. Also, low-level intrinsic contamination in PIF by these microorganisms has been confirmed in some previous outbreaks.
PIF are Not Sterile
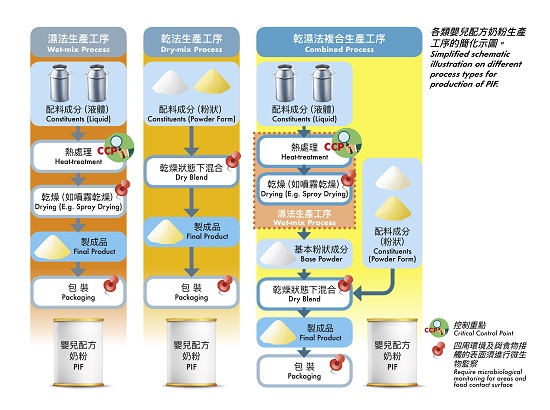
Some people may expect that PIF are sterile products, yet current technology does not achieve the production of sterile PIF. Generally, PIF are manufactured according to three process types: a wet-mix process, a dry-mix process, and a combined process (see illustration). Unlike liquid milk which can be sterilised by heat-treatment in-container (i.e. sterilised milk) or by heat-treatment in a continuous flow system followed by aseptic filling in a sterilised container (i.e. ultra-high temperature treatment milk), PIF have to be produced in dried form where drying or dry blending under non-sterile condition is involved and heat-sensitive ingredients may also be added. It is difficult also to completely inactivate microbial pathogens in the final product as bacterial cells under dry condition usually exhibit increased heat resistance.
Sources of contamination in PIF production can be related to the presence of microorganisms in the processing environment and equipment as well as raw materials. In the wet-mix and the combined processes, the heat-treatment step usually results in significant reduction of the concerned pathogens, and is therefore considered as a critical control point (CCP) in product manufacturing. Contamination of final products, if any, would therefore most likely a result of contamination from processing environment and/or equipment after the drying and during subsequent processing steps. On the other hand for the dry-mix and the combined processes, the microbiological quality of the dry-mix ingredients should meet the requirements for the finished products as there is no further microorganism reduction process during and after mixing of various powdered ingredients. The blending should therefore be done under strict hygienic conditions to avoid contamination. To minimise cross-contamination in all process types, manufacturers should implement ongoing microbiological monitoring programmes for the drying, blending and packaging areas of the plant and for food contact surfaces/equipment.
Recommendations on Reconstituting PIF
Although PIF are not sterile products and can also be contaminated after opening, proper reconstitution prior to feeding could decrease risk from microbiological hazards. The FAO/WHO advises that PIF should be prepared with boiled water no cooler than 70°C, which can significantly inactivate pathogens including Salmonella. Reconstituted PIF should then be cooled to feeding temperature and consumed immediately. Reconstituted PIF that have not been consumed within two hours should be discarded. For high-risk infants (including infants less than two months of age, pre-term infants, low-birth-weight infants (<2.5kg), and immunocompromised infants) who are not breastfed, caregivers should use commercially sterile liquid formulas whenever possible.
Actions Taken by the CFS
Upon receiving notifications of the European Commission that the affected products have been imported into Hong Kong, the CFS instructed the importer concerned to stop sale and remove from shelves the affected batches of the products, and recall the products concerned.
Key Points to Note:
- PIF are not sterile products and may be contaminated with pathogens that can cause serious illness in infants.
- PIF should be prepared with boiled water no cooler than 70°C which can significantly inactivate pathogens including Salmonella.
- Control measures have to be applied by both manufacturers and caregivers to minimise the microbiological risk and to assure the suitability of PIF.
Advice to Caregivers
- Thoroughly clean and sterilise all equipment used for feeding infants and for preparing feeds.
- Beware of the hygienic and safe practices for preparing PIF which are also recommended in prevailing guidelines issued by the FAO/WHO.
Advice to Manufacturers
- Implement preventive measures (such as Good Manufacturing Practice/Good Hygienic Practice and Hazard Analysis and Critical Control Point) as well as monitoring and environmental management programmes.
- Communicate the control measures that the caregivers should follow for the safe preparation, handling and use of PIF on product label.