Risk Assessment Studies
Report No. 41
Nanotechnology and Food Safety
September 2010
Centre for Food Safety
Food and Environmental Hygiene Department
The Government of the Hong Kong Special Administrative Region
Correspondence:
Risk Assessment Section
Centre for Food Safety,
Food and Environmental Hygiene Department,
43/F, Queensway Government Offices,
66 Queensway, Hong Kong .
Email: enquiries@fehd.gov.hk
| Table of Contents |
|---|
| Abstract |
| Objectives |
| Background |
| Properties and Synthesis of Nanomaterials |
| Characteristics of nanomaterials |
| Synthesis of nanomaterials |
| Applications of Nanotechnology in Food Sector |
| Application of nanotechnology in the production of food contact materials |
| Nanoencapsulation of food ingredients and additives |
| Nanostructured food ingredients and additives |
| Other applications |
| Behaviour and Fate of Nanomaterials in the Gastrointestinal (GI) Tract |
| Absorption |
| Distribution |
| Excretion/Elimination |
| Concerns and Health Implications on the Application of Nanotechnology in Food |
| Nanoparticles as indirect sources of food contaminants |
| Alter the absorption profile and metabolism in the body |
| Unknown toxicity of nanoparticles |
| Lack of analytical method/predictive model to evaluate safety of nanoparticles |
| Risk Assessment of Nanomaterials in Food |
| World Health Organization (WHO) and Food and Agricultural Organization of the United Nations (FAO) |
| European Food Safety Authority (EFSA) |
| Health Canada |
| Food and Drug Administration (FDA) |
| Food Standards Australia New Zealand (FSANZ) |
| Availability of nanofood |
| Conclusion and Recommendation |
| Advice to Trade |
| Advice to Public |
| References |
| Annex I |
| Annex II |
Risk Assessment Studies
Report No.41
Nanotechnology and Food Safety
Abstract
While there is currently no internationally agreed definition for nanotechnology, it is usually applied to the process of controlling the size and shape of materials at the atomic and molecular scale. Generally, nanotechnology deals with structures sized between approximately 1 and 100 nanometer (nm) in at least one dimension. Due to the small sizes, nanomaterials exhibit novel features that offer considerable opportunities for the development of innovative products and applications in the food sector to produce potentially safer and healthier products. However, safety issues surrounding the use of nanotechnology in food have raised public concerns.
This study reviewed the basic principles, applications and the potential health implications associated with the use of nanotechnology in the food sector particularly on food and food contact materials incorporated with nanomaterials. In addition, a summary on the current risk assessment approaches adopted by some major countries were provided for references.
Review of the available data showed that at present, there is no tenable evidence that food/food contact materials derived from nanotechnology is any safer or more dangerous than their conventional counterparts. No general conclusion can be made on the safety of nanofood and food contact materials incorporated with nanoparticles. New data and measurement approaches are needed for proper safety assessment of food/food contact materials derived from nanotechnology.
Risk Assessment Studies –
Nanotechnology and Food Safety
OBJECTIVES
The aims of this study are (i) to present the basic principles of nanotechnology; (ii) to identify the potential safety implication associated with the applications of nanotechnology in the food sector; and (iii) to review the strategies for the risk assessment of engineered nanomaterials in food.
BACKGROUND
2. Over the past few decades, the evolution of a number of new technologies has revolutionised the development of the food sector. One of the most notable disciplines is the application of nanotechnology in food.
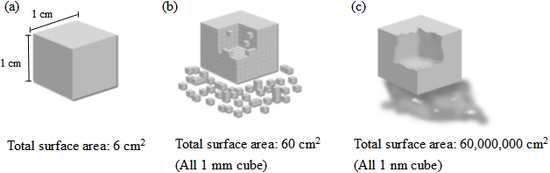
3. While there is currently no internationally agreed definition for nanotechnology, it is usually applied to the process of controlling the size and shape of materials at the atomic and molecular scale. Generally, nanotechnology deals with structures sized between 1 and 100 nanometer (nm) in at least one dimension (Figure 1). 1,2,3 As a consequence of their small sizes, nanoparticles exhibit different physiochemical properties compared to their respective bulk materials. These include changes in optical properties (colour), material strength, conductivity and the surface-to-volume ratio (solubility and reactivity) of the nanoparticles (Figure 2). 5
Figure 1: Examples of nanotechnology derived products.
(a) Nanoparticle: A discrete entity that has all three dimensions in the nanoscale.
(b) Nanotube: A discrete hollow fibre entity which has two dimensions in the nanoscale.
(c) Nanosheet: Nano-object with one external dimension in the nanoscale.
Figure 2: Illustration of the increase in surface area with smaller particle size.
(a) A solid cube with 1 cm on each side has 6 cm2 of surface area.
(b) Volume of 1 cm3 filled with cubes with 1 mm on a side has a total surface area of 60 cm2.
(c) Volume of 1 cm3 filled with cubes with 1 nm on a side has a total surface area of 60,000,000 cm2.
4. Whilst most nanotechnology applications for food and beverages are currently at research and development stage, the presence of nanoparticles in food is not a new phenomenon. Many commonly consumed food ingredients are comprised of proteins, carbohydrates and fats with sizes extending from large biopolymers down to nanoscale. Humans, in fact, have always been exposed to very fine particles in the diet without any harmful effect.6 For the purpose of this review, only matters surrounding nanoparticles/nanomaterials that are deliberately introduced in foodstuffs and their contact materials will be covered. The search strategy is detailed in [Annex I].
PROPERTIES AND SYNTHESIS OF NANOMATERIALS
Characteristics of nanomaterials
5. In general molecules at the surface of a material do not have full quotient of covalent bonds and are in an energetically unstable state. For macroscopic and microscopic materials, with majority of the molecules being in their lowest free-energy state, the proportion of molecules in the material that are in the energetically unstable state is very low and hence it is the properties of the stable molecules that determine the properties of the material. However, as the size of the materials transit from micro-scale to nano-scale, there is a dramatic increase in the surface area of the nanomaterials in which the properties of the more reactive surface molecules dominate and gives rise to the novel properties of the nanomaterials.7
6. Since many more molecules located at the surface are in energetically unstable states, nanomaterials are more reactive compared to the non-nanoscale material. With the high reactivity, almost all types of nanomaterials are capable of catalysing reactions and free nanomaterials tend to agglomerate into bigger particles. The tendency of nanomaterials to agglomerate can be enhanced or hindered by the modification of the surface, for example, in the presence of chemical agents.7 Owning to the specific physiochemical properties of nanoparticles, they are expected to interact with substances such as proteins, lipids, carbohydrates, nucleic acids, ions, minerals and water present in food and biological tissues.8
Synthesis of nanomaterials
7. Nanomaterials can be produced using two building strategies, either a "top-down" or a "bottom-up" approach.
Top-down approach
8. In the top-down approach, nanomaterials are created by breaking up bulk materials using means such as milling to reduce the size of a complex object to the point where this scale reduction begins to alter the very principles it is based upon. However, this method encounters major problem of speed that the slow production rate make it incompatible with the requirement of mass production.3,9
Bottom-up approach
9. The bottom-up approach is radically different, since it involves the building of nanomaterials from individual atoms or molecules that have the capacity to self-assemble like crystal growth. The resources required for building of nanomaterials with the bottom-up approach are considerably reduced since growth and assembly of nanoparticles can be controlled in a single step, and in a natural and self-regulating manner. However, invention and study on the compatibility of different components are needed before any new nanomaterials are synthesised.9
APPLICATIONS OF NANOTECHOLOGY IN FOOD SECTOR
10. With altered properties, nanoparticles are used to produce wide range of materials and products with enhanced quality across different industrial sectors [Annex II]. Although application of nanotechnology in the food sector is only in their infancy, it has been steadily increasing in recent years and is expected to grow rapidly in the coming years.8,10
Application of nanotechnology in the production of food contact materials
11. Food packaging makes up the largest share of current and short-term predicted markets of the use of nanotechnology in the food sector. The addition of nanoparticles into shaped objects and films has been shown to improve properties of the packaging materials with regard to durability,11 temperature resistance,12 flame resistance,13 barrier properties,14 optical properties15 and recycling properties.16
12. Examples of food contact materials currently available in the market include PET (polyethyleneterephthalate) beer bottles with nano-clay gas-barrier, polypropylene food containers with nano-silver for antimicrobial action, nano-zinc oxide containing film for food wrapping and biosensors for monitoring condition of food during storage and transportation.7
Nanoencapsulation of food ingredients and additives
13. Nanoencapsulation is currently the second largest area of nanotechnology application in the food sector.8 It is used as a strategy to harness a controlled delivery system for food ingredients and additives in processed food.10
14. Nanoencapsulation is the technological extension of microencapsulation. Compared to microencapsulation, which has been used by the industry for many years, nanoencapsulation is a new emergent. Nanocarrier systems can be used to mask the unpleasant tastes and flavours of ingredients and additives such as fish oils, to protect the encapsulated ingredients from degradation during processing and storage, as well as to improve dispersion of water-insoluble food ingredients.8 However, current studies on the application of nanoencapsulation mainly address its potential for target delivery of active ingredients of functional food and nutraceuticals.3
Figure 3: Schematic diagram of nanoencapsulation.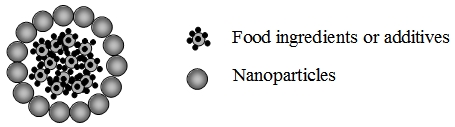
By controlling the surface properties of the nanoparticles, they can be used to encapsulate the bioactive compounds in food. The release of the encapsulating active ingredients from the nanostructure can be triggered by exposure to appropriate environmental conditions (e.g. pH and salt concentration) or through interaction with selected proteins and cells in the body, thus providing a means to deliver the active compounds to the target site.17 Food industry claimed that the addition of nanocapsules to processed foods will improve both the availability and delivery of nutrients, thereby enhancing the nutritional status of food.18 At present, a number of delivery systems are available with a range of encapsulated materials, for example, food additives (e.g. benzoic acid, citric acid) and supplements (e.g. β-carotene and coenzyme-Q10) used in food and beverage products.
16. While the concept of nanodelivery systems can offer the benefit to enhance the absorption, uptake and bioavailability of nutrients and supplements, it also has the potential to alter the distribution of the substances in the body.8 The potential health implications bring about by the use of nanodelivery systems will be discussed in subsequent paragraphs.
Nanostructured food ingredients and additives
17. A major focus of current nanotechnology application in food is the processing and formulation of food ingredients to form nanostructures. The mechanisms commonly used for producing nanostructured food products include nano-emulsions, emulsion bilayers and reverse micelles.19 Examples of nanotextured food products include spreads, ice-creams, yoghurts, mayonnaise, etc. This category of nanofood was being developed with claims that they offer improved taste, texture and consistency19, enhanced bioavailability and allow mixing of "incompatible" ingredients in food matrix.3 It can also be used to produce low-fat nanotextured food products that are as "creamy" as the conventional full-fat equivalent, and hence offers a "healthy" option to the consumer. While nanostructured food ingredients are already available in some health foods, supplements and nutraceutical products, there is currently no clear example of a proclaimed nanostructured food product available on the market.8
Other applications
18. Other indirect applications of nanotechnology in food area include the development of nanosized agrochemicals (such as fertilizers and pesticides) and veterinary medicines.10 An inventory of nanotechnology applications currently on the global food market and associated areas is available on the internet from the Project on Emerging Nanotechnologies.21 Consumers can have update on the latest development of products derived from nanotechnology from this database.
BEHAVIOUR AND FATE OF NANOMATERIALS IN THE GASTROINTESTINAL (GI) TRACT
19. Whether it exists as nanostructured food ingredient, nanocarrier or nano-sized particles incorporated in food packaging, human exposure to nanomaterials present in food or food contact materials through ingestion. The whole cascade of events including absorption, distribution, metabolism and excretion/elimination occur following ingestion determines the internal exposure and toxicity of these substances. However, due to the interactions of nanomaterials with surrounding matrix and unexpected effects resulting from this, little is known regarding the behaviour and fate of nanomaterials in the GI tract.7
Absorption
20. Nanomaterials present in food may be readily absorbed from the GI tract. Translocation of nanomaterials through the epithelium of the intestinal wall depends on their physiochemical properties, for example, size, surface charge, lipophilicity/hydrophilicity, presence/absence of a ligand, and physiology of the intestinal tract.22 Oral administration of gold nanoparticles to mice showed that gastrointestinal uptake of these particles increased with diminishing size23 and smaller particles are absorbed more readily and faster than larger ones.24
21. However, it is also possible that the ingested nanomaterials may not remain in a free form in the lumen due to transformations such as agglomeration, aggregation, adsorption or binding with other food component and hence are not readily available for translocation through the intestinal wall. Currently, only limited information is available on the absorption of nanomaterials after ingestion.8 The present absorption studies have mostly been performed on metal and plastic nanomaterials not intended for food use. The translocation of the nano-sized particles potentially used as food components through the GI tract remains to be explored.7
Distribution
22. Upon contact with the intestinal sub-mucosal tissue, the ingested nanomaterials can enter the capillaries, which will carry them through the portal circulation to the liver, or they enter the lymphatic system via the thoracic ducts.
23. Experimental data demonstrated that the distribution of nanoparticles after oral administration is dependent upon particle size. Smaller-sized nanoparticles have a more widespread tissue distribution to organs like kidney, liver, lungs and brain while the bigger particles (28 nm and 58 nm) remain almost solely inside the GI tract.23 Studies have been performed on the ability of nanoparticles to penetrate the placental barrier. There is also information that certain nanomaterial (C60 fullerene) can pass across the placenta.25 However, due to the inconsistent results of some in vitro26and animal studies25, no general conclusion on the penetration power of nanoparticles across the placental barrier can be made. There is no information on whether nanomaterials are transferred into milk.7
Excretion/elimination
24. There is very limited information on the excretion of absorbed nanomaterials. Animal study feeding rats with radioactive iridium nanoparticles (192Ir) showed that the ingested nanoparticles were not substantially uptake through the GI tract and were rapidly eliminated via faeces within 2-3 days. No significant translocation of the nanoparticles from the GI tract to other organs through the blood was observed.27 A positive surface charge was also found to increase both urinary and faecal excretion.28
CONCERNS AND HEALTH IMPLICATIONS ON THE APPLICATION OF NANOTECHNOLOGY IN FOOD
25. The modified properties of nanoparticles provide the public with potentially safer and healthier food. However, safety issues surrounding the application of nanotechnology in food have also attracted public concerns.
Nanoparticles as indirect sources of food contaminants
26. The growing use of materials, products and applications of nanotechnology may pose new indirect sources of food contamination with nanoparticles. Such risk of exposure may arise from the use of nano-sized pesticides and veterinary medicines, contact of food with nanoparticulate-based coatings during preparation or processing, or potential migration of nanoparticles from food packaging.10 Because of the small sizes, these nanoparticles may enter the food chain undetected, accumulate within tissues and organs, and can be taken up by individual cells.3 There is also concern that the introduction into food of nanoparticles designed to carry dietary supplements could also lead to introduction of foreign substances into the bloodstream.10
Alter the absorption profile and metabolism in the body
27. The processing of food ingredients into nano-size may make them different from those that exist naturally. As nano-sized particles have been shown to have a greater ability to cross the gut wall compared to microparticles of the same kind, their enhanced absorption and bioavailability would give rise to higher internal exposure or with higher plasma concentrations. This change in properties of the nano-scale processed foods may also alter the way of how the food ingredients behave upon breakdown with the gut and, as a consequence, how they are treated in the GI tract.10
28. It is not only the nanomaterials itself that may trigger biological effects. These nano-sized materials may interact with proteins and other compounds nearby and act as carriers of these substances into different biological tissues. It has been suggested that the carrier potential of nanomaterials might impact the absorption of molecules, for example by introducing unintended molecules across the GI tracts, leading to unintended effects.8
29. It has been evidenced that the consumption of nano-sized food ingredients has the potential to alter body metabolism of experimental animals. Oral administration of nano-calcium-enriched milk to ovariectomised rats has been shown to alter the calcium metabolism. Urinary excretion of two chemical components, deoxypyridinoline and hydroxyproline, were found to decrease in rats ingested with the nano-calcium containing milk indicating a reduction of bone resorption and increased bone formation. However, such change in the bone regeneration was accompanied by an increased excretion of calcium in urine and the mechanisms responsible for these remains unclear.29 At present, there is currently not much information regarding metabolism/biotransformation of nanomaterials after oral administration in human model.
30. The changed absorption profile and body metabolism creates complications for the exposure assessments of these novel materials within the body.
Unknown toxicity of nanoparticles
Oral exposure studies
31. Owning to their increased surface reactivity and potentially altered bio-kinetics, nanoparticles may have a toxicity that deviates from that of their bulk equivalents.8,30 However, studies on the toxicology of nanomaterials are not common and much of the research address primarily the inhalation exposure10 and occupational hazards associated with the manufacture and handling of nanostructured materials.8
32. While for oral studies, so far they are limited to acute dosing, long-term studies have not been conducted7. The FAO/WHO Expert Meeting on the Application of Nanotechnologies in the Food and Agriculture Sectors: Potential Food Safety Implications has pointed out that the quality of many of the published oral toxicity studies is questionable, severely limiting the use of the information. The current state of knowledge does not permit reliable prediction of the toxicological characteristics of any give nanomaterials from data on other nanomaterials or from a consideration of the characteristics of the nanomaterials itself.8 No data on genotoxicity, or on possible carcinogenesis and teratogenicity, is available for nanoparticles yet.8,31
Chemical analysis
33. The different physiochemical properties of nanomaterials compared to conventional dissolved and macroscale chemicals imply that their toxicokinetic and toxicity profiles of nanoparticles cannot be fully inferred by extrapolation from data on their equivalent non-nanoforms.7 However, currently only a few available studies have compared the toxicity of nanoformulated and conventional form of the sample chemical species and these data are insufficient upon which to base any conclusion about the toxicity of nanomaterials.
In vitro studies
34. There is a wealth of in vitro studies of nanomaterials in human or animal cells and wide range of nanomaterials, concentrations and exposure times have been studied.
Potential to cross cellular barriers and cause oxidative damage
35. In vitro studies have demonstrated that free engineered nanoparticles are capable of crossing cellular barriers 32,33 and exposure to ultrafine particulate pollutants (<100 nm) can lead to increased production of oxyradicals and, consequently, oxidative damage to the cell.34
Potential for brain-cell damage
36. Exposure of mouse microglia, specialised cells that protect the brain from harmful external stimuli, to titanium dioxide (TiO2) nanoparticles has been shown to trigger rapid and long-lasting defensive response. Although the microglia generate reactive oxygen species (ROS) as a defensive mechanism, a prolonged release of these ROS can be harmful to the brain by mechanisms similar to the cause of neuronal damage in certain neurodegenerative diseases, including Parkinson's and Alzheimer's.35
Potential to cause impairment of DNA replication and transcription
37. In vitro study on human epithelial cell cultures using silicon dioxide (SiO2) nanoparticles demonstrated that particles smaller than 70 nm could enter cell nuclei. The study also found protein accumulation in the nuclei and indication for impairment of DNA replication and transcription.36 Although SiO2 is used as an additive to food and food packaging, it is not known whether its intake through the gastrointestinal route, along with other food substances, will lead to comparable effects in vivo.10
38. Although potential toxicity of certain nanomaterials has been demonstrated in a number of in vitro studies, there are limitations of such studies that the toxicity of the nanomaterials in food could not be totally reflected. Typical problems have been administration of physiologically non-relevant doses, aggregation of particles, direct exposure of the cells to nanomaterials and the uncertainty in the interaction between nanomaterials and other food components.
39. Despite the limited information on the toxicity of nanofood, study conducted by the House of Lords Select Committee on Science and Technology of UK received no evidence of instances where ingested nanomaterials have harmed human health.37
Lack of analytical method/predictive model to evaluate safety of nanoparticles
40. In face of the novelty of the nanoparticles, our conventional knowledge about health effects of chemical and materials, which is based on their chemical and physical properties, may not be applicable when dealing with nanoproducts.30 Methods for identifying and establishing the relevant hazards of different chemicals were developed for molecular form of materials, and nanoscale materials may behave differently, the ability of the available analytical methods may not be sufficient to support the decision about the biological effects of nano-sized particles.38
41. At present, a number of analytical tools are available for the qualitative and quantitative characterisation of nanomaterials. These include microscopy and related techniques such as atomic force microscopy (AFM) to observe properties (the state of aggregation, dispersion, sorption, size, structure and shape) of the nanomaterials and chemical analytical methods to detect the presence of these materials.7,39 Due to the diverse variety of nanomaterials, there are many different ways for analysis and there is no single technique that can be used for all situations and therefore a combination of techniques is usually necessary.7
42. As the properties of the nanomaterials may depend on the surrounding matrix, there are limitations for the precise detection, quantification and characterisation of nanomaterials in food. For instance, detection by electron microscopy is only possible if the number of the nanomaterials is sufficiently high to find a detectable number of nanomaterials in the matrix and detection may also be hindered by interactions with solutes or cell constituents that obscure clear analytical signals.7 In some other cases, engineered nanomaterials may not be distinguishable from naturally occurring variants of the same material. The limited number of standardised reference materials for nanomaterials is another limitation on precise and reproducible detection and quantification of the nano-sized materials in food.7
43. While for some chemical analytical methods, artificial losses during preparatory steps and the analytical limits of these methods make it not suitable for precise quantification of nanomaterials present in food.7
44. In summary, there are methods available to detect and analyse nanomaterials under certain conditions, there are, however, no routine methods available for analysing nanomaterials in the food area. In view of the present difficulties in detection of nanomaterials in food matrices, knowledge regarding the presence of nanomaterials in food products relies on information provided by the industry.7
RISK ASSESSMENT OF NANOMATERIALS IN FOOD
45. A number of national and international advisory committees have recommended strategies for the risk assessment of nanomaterials.40,41 However, there is currently no comprehensive guidance developed particularly for the safety assessment of nanomaterials in food. A difficulty in giving detailed specific risk assessment guidance in the area is the lack of sufficient data and information which would allow for a comprehensive understanding of potential hazards of nanomaterials.7
World Health Organization (WHO) and Food and Agricultural Organization of the United Nations (FAO)
46. WHO commented that as for all new materials used in food and food processing, the potential health and environmental risks of nanoscale materials need to be assessed before they are introduced into food.1
47. Given the increased global interest in the use of nanotechnology and concerns on the potential food safety implications, FAO and WHO convened the "FAO/WHO Expert Meeting on the Application of Nanotechnologies in the Food and Agriculture Sectors: Potential Food Safety Implications" to provide information on what was currently known about potential food safety risks, identify potential food safety implications associated with applications of nanotechnologies in the food sector as well as to review current risk assessment methodologies to evaluate the safety of nanomaterials in the food-chain.42
48. The Expert Meeting acknowledged that current risk assessment approaches (hazard identification, hazard characterisation, exposure assessment and risk characterisation) used by FAO/WHO and Codex are suitable for nanomaterials used in food and emphasised that additional safety concerns may arise owing to the characteristic properties of nanomaterials. The experts have also identified the challenges of risk assessment (e.g. knowledge gaps, need of improved methods to detect nanoparticles in complex matrices, etc.) and agreed that FAO/WHO should continue to review its risk assessment strategies in order to address the specific emerging issues associated with the application of nanotechnologies in the food chain. It was also agreed that the development of validated testing methods and guidance would help to address specific data gaps. The experts recommended the FAO/WHO should encourage the innovative and interdisciplinary research that may lead to novel risk assessment strategies for the application of nanotechnologies in food.8
European Food Safety Authority (EFSA)
49. EFSA is of the view that given the novelty of nanotechnology, the safety of possible food and feed applications needs to be assessed and a need for risk assessment is expected in the context of 1) the authorisation of regulated substances; 2) the presence of nanoparticles as contaminants in food and feed; and 3) replies to general request such as whether the application of nanotechnologies in food and production leads to changes in nutritional value or bioavailability.43
50. On the request of the European Commission, EFSA provided a scientific opinion on the potential risks arising from nanoscience and nanotechnologies on food and feed safety in February 2009. The Opinion stated that the formulation at the nanosize may change the physiochemical characteristics of materials as compared to the dissolved and micro/macro forms of the same substance. The small size, high surface-to-mass ratio and surface reactivity of the nanomaterials are important properties, both for new applications and in terms of the associated potential health and environmental risks.7
51. Risk assessment paradigm was considered applicable for nanomaterials and has to be performed on a case-by-case basis. Current toxicity-testing approaches used for conventional materials were a suitable starting point for risk assessment of nanomaterials. However, EFSA specifically concluded that although case-by-case evaluation of specific nanomaterials may be possible, uncertainties for risk assessment of nanotechnologies and their possible applications in the food and feed area arise due to presently limited information in several areas. Specific uncertainties applied to the difficulty to characterise, detect and measure nanomaterials in food/feed and biological matrices and the limited information available in relation to aspects of toxicokinetics and toxicology, as well as the lack of optimal methods for testing nanomaterials. There is limited knowledge of exposure from possible applications and products in the food and feed area. The current usage levels of nanomaterials in the food and feed area is unknown. The limited database on nanomaterials assessments should be considered in the choice of appropriate uncertainty factors in risk characterisation step.7
52. EFSA has also made several recommendations, in particular, actions should be taken to develop methods to detect and measure nanomaterials in food and biological tissues, to survey the use of nanomaterials in food area, to assess the exposure in consumers, and to generate information on the toxicity of different nanomaterials.7
53. As the impact of nanotechnology on human health and the environment is still not fully known, in order to clarify some of the issues regarding the application of nanotechnology, the European Commission (EC) released a recommendation to the Member States to adopt a Code of Conduct for Nanosciences and Nanotechnologies in February 2008 to govern research in the fields of nanosciences and nanotechnologies.44
Health Canada
54. As a result of consultations from a broad range of interested parties, Health Canada has developed an overall draft framework approach to the products and substances generated by the application of nanotechnology, including food, but the framework has yet to be officially adopted by Health Canada. The use of nanomaterials as a component of food or used to produce food products are currently subject to the same health and safety regulations that apply to conventional materials. Health Canada considers the Food Additive Regulations, Novel Food Regulations and Food and Packaging Materials Regulations to be relevant for food products that is using or derived from nano-objects.
55. In Canada, any new additive and food products which fall under the definition of "novel food" are subject to pre-market safety assessments before being introduced to the food supply. In both cases, the assessment focuses on food safety and considers the toxicological aspects as well as relevant microbiological and/or nutritional factors. As oppose to food additives or novel foods, packaging materials intended for use with foods may be submitted voluntarily for a pre-market safety assessment of their chemical safety. Health Canada is envisaging taking a case-by-case approach to the safety assessment of food products containing or using nano-objects which will allow experience to be built for each type of products reviewed and thereby help better defining adequate requirements for industry. However, Health Canada calls for precaution when it comes to new materials which have not been previously assessed in their conventional form and for nanomaterial-containing food products for which pre-market safety assessments are not required.
US Food and Drug Administration (FDA)
56. According to FDA, products are regulated based on their statutory classification rather than the technology they employ.38 As such, the safety of products containing nanoscale materials was assessed similarly as their respective bulk materials. For products subject to premarket authorisation such as food and colour additives, FDA can require manufacturers to provide the necessary scientific information to review the safety and effectiveness of the products. For products not subject to premarket authorisation by FDA, such as dietary supplements and food generally recognised as safe, manufacturers generally are not required to submit data to FDA prior to marketing. However, manufacturers are still responsible for ensuring that the products they market are safe.38
57. In 2006, FDA formed the Nanotechnology Task Force to help assess the question on adequacy and application of the regulatory authorities posed by the use of nanotechnology. The Task Force commented in its initial report that the available information does not suggest that all materials with nanodimensions will be hazardous. Furthermore, if all nanoscale materials are compared to all non-nanoscale materials, it is not apparent that the nanoscale materials as a group would have more inherent hazard. The report also indicated that nanoscale materials present regulatory challenges similar to those posed by products using other emerging technologies and highlighted the need for timely development of a transparent, consistent, and predictable regulatory pathway for nanotechnology.38
Food Standards Australia New Zealand (FSANZ)
58. FSANZ recognises that very small particles of matter can behave differently to larger particles of even the same matter and that care does need to be taken to ensure that small particles in food are just as safe as larger ones. The size of particles in food however, is only one of a number of considerations relevant to ensuring the safety of food. When FSANZ assesses the safety of foods or food ingredients, it examines a wide range of scientific evidence that establishes that it is safe to consume. Besides, any new food substances intended for commericialisation require premarket safety assessment and approval, before they can legally be supplied.2
59. If FSANZ receives an application for a new type of engineered nanometre scale particle in food, it would be assessed depending on the type of substance or food and the standards that applies (e.g. whether it is a processing aid, food additive, novel food or novel food ingredient) to address if there is any added risk to human health and safety due to the characteristics of newly engineered nanoparticles.2
AVAILABILITY OF NANOFOOD
60. At present, such foods are known to be available on the global market, mainly through internet trading.45 We are not aware of any country conducting risk assessment on specific food products produced using nanotechnology.
CONCLUSION AND RECOMMENDATION
61. There is currently no internationally agreed definition for "nanotechnology". A clear and internationally harmonised description of the technology would help to define the scope for safety assessment and regulation on the application of nanotechnology in food.
62. At present, there is no tenable evidence that food or food contact materials derived from nanotechnology is any safer or more dangerous than their conventional counterparts. No general conclusion can be made on the safety of nanofood and food contact materials incorporated with nanomaterials. Most scientific committees that have reviewed the applications of nanotechnology concluded that while consumers are likely to benefit from this technology, new data and new measurement approaches may be needed to ensure that the safety of products using nanotechnology are properly assessed.1
63. Many regulatory authorities have evaluated their framework of regulation and approval of food ingredients to ensure the currently adopted systems can fully encompasses the use of nanotechnology in food and food-contact materials. The approaches for safety evaluation of nanomaterials vary from country to country but presumably follow similar pathways to those used for other materials proposed for use in food and food contact materials.1
64. As methods for detection and characterisation of nanomaterials in food were not readily available, in many instances the claimed nanoscale character of the applications could not be verified. Knowledge regarding the presence of nanomaterials in food products relies on information provided by the industry, producers and marketing organisations.
ADVICE TO TRADE
a) Traders should ensure the products on sale are safe for human consumption.
b) Do not sell nanomaterials that have not undergone safety assessment.
ADVICE TO PUBLIC
a) Maintain a balanced diet.
b) Buy food from reliable suppliers.
REFERENCES
1 World Health Organization (WHO). Nanotechnology. International Food Safety Authorities Network (INFOSAN) Information Note No. 01/2008. (Rev 1. March 2008). 2008. [cited 9 September 2008]. Available from:
http://www.who.int/entity/foodsafety/fs_management/No_01_nanotechnology_Feb08_en_rev1.pdf
2 Food Standards Australia New Zealand (FSANZ). Small Particles, Nanotechnology and Food. June 2008. [cited 14 August 2008]. Available from: URL:
http://www.foodstandards.gov.au/newsroom/factsheets/factsheets2008/smallparticlesananote3923.cfm
3 Hsieh, YHP and Ofori JA. Innovation in food technology for health. Asia Pacific Journal of Clinical Nutrition 2007; 16(Suppl 1): 65-73.
4 European Food Safety Authority (EFSA). Nanotechnology. 2008. [cited 14 August2008]. Available from: URL:
http://www.efsa.europa.eu/EFSA/KeyTopics/efsa_locale-1178620753812_Nanotechnology.htm
5 ETC Group. The big down: Atomtech – Technologies converging at Nano-scale. January 2003. [cited 9 September 2008]. Available from: URL:
http://www.etcgroup.org/documents/TheBigDown.pdf
6 Food Safety Authority of Ireland (FSAI). The Relevance for Food Safety of Application of Nanotechnology in the Food and Feed Industries. 2008. [cited 16 April 2009]. Available from: http://www.fsai.ie/publications/reports/Nanotechnology_report.pdf
7 European Food Safety Authority (EFSA). Scientific Opinion of the Scientific Committee on the Potential Risks Arising from Nanoscience and Nanotechnologies on Food and Feed Safety. 10 February 2009. [cited 16 April 2009]. Available from:
http://www.efsa.europa.eu/EFSA/Scientific_Opinion/sc_op_ej958_nano_en,0.pdf?ssbinary=true
8 Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO). Report of Joint FAO/WHO Expert Meeting on the Application of Nanotechnologies in the Food and Agriculture Sectors: Potential Food Safety Implications. [cited 18 December 2009] Available from:
http://www.fao.org/ag/agn/agns/files/FAO_WHO_Nano_Expert_Meeting_Report_Final.pdf
9 Labrune JC and Palmino F. Nanowires. In: Dupas C, Houdy P and Lahmani M. Nanoscience, Nanotechnologies and Nanophysics. Berlin: Springer; 2004. p. 325-79.
10 Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L et al. Applications and implications of nanotechnologies for the food sector. Food Additives and Contaminants 2008; 25 (3): 241-58.
11 Wang KK, Koo CM and Chung IJ. Physical properties of polyethylene/silicate nanocomposite blown films. Journal of Applied Polymer Science 2003; 89: 2131-6.
12 Kotsilkova R, Petkova V and Pelovski Y. Thermal analysis of polymer-silicate nanocomposites. Journal of Thermal Analysis. Calorimetry 2001; 64: 591-8.
13 Ray SS, Maiti P, Okamoto M, Yamada K and Ueda K. New polylactide/layered silicate nanocomposites. 1. Preparation characterisation properties. Macromolecules 2002; 35: 3104-10.
14 Xu B, Zheng Q, Song Y and Shangguan Y. Calculating barrier properties of polymer/clay nanocomposites: Effects of clay layers. Polymer 2006; 47: 2904-10.
15 Wan C, Qiao X, Zhang Y and Zhang Y. Effect of different clay treatment on morphology mechanical properties of PVC-clay nanocomposites. Polymer Testing 2003; 22: 453-61.
16 McGlashan SA and Halley PJ. Preparation characterisation of biodegradable starch-based nanocomposite materials. Polymer International 2003; 52: 1767-73.
17 Sanguansri P and Augustin MA. Nanoscale materials development – a food industry perspective. Trends in Food Science and Technology 2006; 17: 547-56.
18 Scrinis G and Lyons K. The emerging nano-corperate paradigm: nanotechnology and the transformation of nature, food and agri-food systems. International Journal of Sociology of Food and Agriculture 2007; 15(2): 22-44.
19 Weiss J, Takhistov P and McClements DJ. Functional Materials in Food Nanotechnology. Journal of Food Science 2006; 719: R107-16.
20 International Union of Food Science & Technology (IUFoST). Nanotechnology and Food. IUFoST Scientific Information Bulletin. December 2007. [cited 10 September 2008]. Available from:
http://www.iufost.org/reports_resources/bulletins/documents/IUF.SIB.Nanotechnology.pdf
21 The Project on Emerging Nanotechnologies. An inventory of nanotechnology-based consumer products currently on the market. 2008. [cited 3 November 2008]. Available from: URL: http://www.nanotechproject.org/inventories/consumer/
22 Des Rieux A, Fievez V, Garinot M, Schneider YJ and Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. Journal of Control Release 2006; 116(1):1-27.
23 Hillyer JF and Albrecht RM. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. Journal of Pharmaceutical Science 2001; 90(12): 1927-36.
24 Szentkuti L. Light microscopical observations on luminally administered dyes, dextrans, nanospheres and microspheres in the pre-epithelial mucus gel layer of the rat distal colon. Journal of Controlled Release 1997; 46(3): 233-42.
25 Tsuchiya T, Oguri I, Yamakoshi YN and Miyata N. Novel harmful effects of [60]fullerene on mouse embryos in vitro and in vivo. FEBS Letter 1996; 393(1): 139-45.
26 Myllynen PK, Loughran MJ, Vyvyan Howard C, Sormunen R, Walsh AA and Vahakangas KH. Kinetics of gold nanoparticles in the human placenta. Reproductive Toxicology 2008; 26(2): 130-7.
27 Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. Journal of Toxicology and Environmental Health Part A 2002; 65: 1513-30.
28 Balogh L, Nigavekar SS, Nair BM, Lesniak W, Zhang C, Sung LY, et al. Significant effect of size on the in vivo biodistribution of gold composite nanodevices in mouse tumor models. Nanomedicine 2007; 3(4): 281-96.
29 Park HS, Ahn J and Kwak HS. Effect of nano-calcium-enriched milk on calcium metabolism in ovariectomised rats. Journal of Medicinal Food 2008; 11(3): 454-9.
30 Dingman J. Nanotechnology: its impact on food safety. Journal of Environmental Health 2008; 70(6): 47-50.
31 Bouwmeester H, Dekkers S, Noordam MY, Hagens WI, Bulder AS, de Heer C, etc. Review of health safety aspects of nanotechnologies in food production. Regulatory Toxicology and. Pharmacoogy 2009; 53: 52–62.
32 Koch AM, Reynolds F, Merkle HP, Weissleder R and Josephson L. Transport of surface-modified nanoparticles through cell monolayers. Chemistry and Biochemistry 2005; 6: 377-45.
33 Geiser M, Rothen-Rutishauser B, Kapp N, Schürch S, Kreyling W, Schulz Y et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environmental Health Perspectives 2005; 113: 1555-60.
34 Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental Health Perspectives 2003; 111: 455-60.
35 Thrall L. Study links TiO2 nanoparticles with potential for brain-cell damage. Environmental Science and Technology 2006; 40(14): 4326-7.
36 Chen MA and Mikecz von A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Experimental Cell Research 2005; 305: 51-62.
37 House of Lords Science and Technology Committee. Nanotechnologies and Food. 1st Report of Session 2009-10. January 2010. [cited 20 January 2010]. Available from: http://www.publications.parliament.uk/pa/ld200910/ldselect/ldsctech/22/22i.pdf
38 US Food and Drug Administration. Nanotechnology – a report of the US Food and Drug Administration Nanotechnology Task Force. July 2007. [cited 11 September 2008]. Available from: http://www.fda.gov/nanotechnology/taskforce/report2007.pdf
39 Tiede K, Boxall ABA, Tear SP, Lewis J, David H and Hassellov M. Detection and characterisation of engineered nanoparticles in food and the environment. Food Additives and Contaminants 2008; 25(7):795-821.
40 Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Risk assessment of products of nanotechnologies. 19 January 2009. [cited 20 April 2009]. Available from: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_023.pdf
41 Scientific Committee on Consumer Products (SCCP). Preliminary Opinion on Safety of Nanomaterials in Cosmetic Products. 19 June 2007. [cited 20 April 2009]. Available from: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_099.pdf
42 World Health Organization (WHO). Joint FAO/WHO Expert Meeting on the Application of Nanotechnologies in the Food and Agriculture Sectors: Potential Food Safety Implications. Scope and Objectives. 2009. [cited 17 April 2009]. Available from:
http://www.who.int/foodsafety/fs_management/meetings/Nano_Scope_Objectives.pdf
43 European Food Safety Authority (EFSA). Nanotechnology. 2008. [cited 14 April 2009]. Available from: URL:
http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1178680051172.htm
44 European Public Health Alliance (EPHA). Update – EFSA calls for scientific data on applications of nanotechnology and nanomaterials used in food and feed. February 2008. [cited 11 September 2008]. Available from: URL:
http://www.epha.org/a/2919
45 Food Safety Authority of Ireland (FSAI). The relevance for food safety of applications of nanotechnology in the food and feed industries. 2008. [cited 15 December 2009]. Available from: URL: http://www.nanowerk.com/nanotechnology/reports/reportpdf/report119.pdf
Annex I
Literature Search Strategy
To identify literature relevant to the research questions, multiple database searches were conducted using internet search engines and bibliographical databases. Academic literature was obtained via ISI Web of Science and Google Scholar. Searches for internet resources were conducted through the search engines Metacrawler and Google.
All search terms were limited to publication dates ranging from 2000-2009 (inclusive) and with English language version available. Abstracts of identified articles were examined and reference related to the application, potential risk, as well as safety assessment of nanofood was retrieved for inclusion in the review. A summary of the database searched and key terms used were set out below.
| Database / Search engine | Search terms | Relevant publications retrieved | ||||
|---|---|---|---|---|---|---|
|
ISI Web of Science |
Nanotechnolog* |
AND |
concern* |
NOT |
2 |
|
|
food* |
28 |
|||||
|
risk* |
5 |
|||||
|
safety |
10 |
|||||
|
toxicity |
1 |
|||||
|
Nanoscience* |
food* |
2 |
||||
|
Nanoparticle* |
food* |
7 |
||||
|
health effect* |
1 |
|||||
|
risk* |
3 |
|||||
|
safety |
2 |
|||||
|
testing |
2 |
|||||
|
toxicity |
environment |
13 |
||||
|
Nanomaterial* |
health effect* |
2 |
||||
|
risk* |
5 |
|||||
|
safety |
2 |
|||||
|
testing |
1 |
|||||
|
toxicity |
environment |
7 |
||||
|
Google Scholar |
Nanotechnology / Nanotechnologies |
food / foods |
29 |
|||
|
risk / risks |
1 |
|||||
|
safety |
4 |
|||||
|
Nanoparticle / Nanoparticles |
food / foods |
2 |
||||
|
safety |
1 |
|||||
|
toxicity |
1 |
|||||
|
Nanomaterial / Nanomaterials |
food /foods |
1 |
||||
|
health effect / health effects |
3 |
|||||
|
safety |
1 |
|||||
* represents any groups of character, including no character.
In addition, references were also made to relevant publications from national and international food safety authorities such as the World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO), European Food Safety Authority (EFSA), U.S. Food and Drug Administration (FDA), Food Standards Australia New Zealand (FSANZ), Health Canada and International Life Science Institute (ILSI). Reference lists of the retrieved documents were hand searched to identify additional publications. Items that have been quoted or made direct reference to were listed in the reference section.
Officials from relevant food safety authorities were also consulted on the approaches adopted for the risk assessment of food derived nanotechnology.
Examples of Nanotechnology Applications
| Products | Nanotype | Commercial claims |
|---|---|---|
|
Paints |
Carbon nanotubes |
Paint added with nanotubes to block radio transmission and cell phone signals |
|
Refrigerator and washing machine |
Silver nanoparticles |
Silver particles of nano-size confer a strong antimicrobial effect which is desirable for the coating of the inner surfaces of refrigerator and washing machine |
|
Sun screen |
Titanium dioxide nanoparticles |
Titanium dioxide nanoparticles have a comparable UV protection property as the bulk material but lose the cosmetically undesirable whitening as the particle size is decreased |
|
Clothing |
Nano fibre |
Fabric that resists spills, repels stains, wicks away moisture and resists static |
|
Tennis racket |
Carbon nanotubes |
Tennis racket weaved from titanium and carbon nanotubes provide enhanced stiffness and return more energy to the ball |
|
Nutrition cream (cosmetics) |
Gold nanoparticles |
Gold nanoparticles equip good biological compatibility. The use of this type of particles in cosmetic products can improve absorption of the active ingredients |