Risk Assessment Studies
Report No. 30
Chemical Hazard Evaluation
DIETARY EXPOSURE TO BENZOIC ACID FROM PREPACKAGED NON-ALCOHOLIC BEVERAGES OF SECONDARY SCHOOL STUDENTS
October 2007
Centre for Food Safety
Food and Environmental Hygiene Department
The Government of the Hong Kong Special Administrative Region
Correspondence:
Risk Assessment Section
Centre for Food Safety
Food and Environmental Hygiene Department
43/F, Queensway Government Offices,
66 Queensway, Hong Kong
Email: enquiries@fehd.gov.hk
Table of Contents |
Page |
|
Executive Summary |
2 |
|
Objectives |
5 |
|
Introduction |
5 |
|
Hazard Identification |
7 |
|
Hazard Characterisation |
8 |
|
Exposure Assessment |
11 |
|
Risk Characterisation |
19 |
|
Limitations of the Exposure Assessment Study |
26 |
|
Conclusion and Recommendation |
27 |
|
References |
29 |
Risk Assessment Studies
Report No. 30
DIETARY EXPOSURE TO BENZOIC ACID FROM PREPACKAGED NON-ALCOHOLIC BEVERAGES OF SECONDARY SCHOOL STUDENTS
Executive Summary
The Centre for Food Safety (CFS) has conducted a study on benzoic acid in prepackaged beverages in order to evaluate the dietary exposure to this chemical in secondary school students and assess the associated health risks.
Study on benzoic acid levels in prepackaged beverages
During August and September 2006, the CFS sampled a total of 211 prepackaged beverages, including (i) soft drink (both diet/light and regular types), (ii) fruit juice, (iii) soy milk, (iv) Chinese tea and (v) coffee/tea from local retail markets. Laboratory analysis for benzoic acid was conducted by the Food Research Laboratory of the CFS.
The results showed that benzoic acid was detected in 36 (17.1%) beverage samples, including soft drink and fruit juice, with concentrations ranging from 51 to 580 mg/kg.
Exposure assessment of benzoic acid from prepackaged beverages of secondary school students
he dietary exposure to benzoic acid was estimated using the local food consumption data obtained from secondary school students in 2000 and the concentrations of benzoic acid in beverages taken from the local market.
Soft drink contributed to about 80% of the benzoic acid exposure from prepackaged beverages of secondary school students.
The average dietary exposure to benzoic acid from prepackaged beverages of secondary school students was 0.31 mg/kg bw/day (ranging from 0.17 to 0.41 mg/kg bw/day). For high consumers (95 th percentile), the dietary exposure to benzoic acid was 0.97 mg/kg bw/day (ranging from 0.58 to 1.30 mg/kg bw/day). Both exposure levels fell well below the Acceptable Daily Intake (ADI) of 0 - 5 mg/kg bw established by Joint FAO /WHO Expert Committee on Food Additives (JECFA), which accounted for 6.1% and 19.3% of the ADI in average and high consumers of secondary school students respectively.
However, based on the assumption that consumers had specific brand loyalty towards the beverage sample with the highest detected benzoic acid level in this study, the exposure to benzoic acid in some high consumers (boys aged 11-14 years) would exceed the ADI by 28.0%. This highlighted a potential public health concern for secondary school students consuming beverages with high levels of benzoic acid.
Conclusion
Upon normal consumption, the dietary exposures to benzoic acid from prepackaged beverages in both average and high consumers of the secondary school students were well below the ADI, suggesting secondary school students were unlikely to experience the adverse effects of benzoic acid from prepackaged beverages.
Advice to trade
- Observe the regulatory requirements under the Public Health and Municipal Services Ordinance, Cap. 132, regarding the use and labelling of benzoic acid.
- Observe good manufacturing practices to limit the use of food additives i.e. benzoic acid in food and drinks to the lowest possible level necessary to accomplish its desired effect.
Advice to public
- Maintain a varied and balanced diet so as to avoid excessive exposure to benzoic acid and other food additives from a small range of food items.
Advice to benzoic acid sensitive individuals
- Read food labels carefully before purchasing or consuming prepackaged food to see whether the food contains benzoic acid.
- Seek medical professionals' advice when necessary.
Objectives
This study aims at estimating the dietary exposure to benzoic acid from prepackaged beverages of secondary school students in Hong Kong and assessing the associated health risks.
Introduction
|
2.
|
Benzoic acid is one of the oldest chemical preservatives used in food, cosmetics and drugs. In Hong Kong, benzoic acid (International Numbering System Number, INS No. 210) and its sodium (INS No. 211), potassium (INS No. 212) and calcium salts (INS No. 213) (collectively referred to as benzoic acid for the purposes of this study) are permitted preservatives under the Preservatives in Food Regulations (Cap. 132 sub. leg. BD). As stipulated in the Regulations, benzoic acid is permitted in specified food items including beer, fruit juices and soy sauce, with maximum permitted levels ranging from 70 mg/kg to 10,000 mg/kg in different types of food. For non-alcoholic beverages, the maximum permitted levels range from 160 mg/kg in soft drinks to 2,000 mg/kg in grape juice products intended for sacramental use.
|
|
3.
|
However, adverse health impact regarding the intake of benzoic acid has aroused public concern recently. Media reported that scientists had produced further evidence suggesting the ability of sodium benzoate to cause DNA damages 1 and neuro-degenerative diseases such as Parkinson's disease.
|
|
4.
|
The concern about overexposure to benzoic acid was considerable especially in the young population. Children were at higher risks as they have high energy intake per kg body weight and different dietary patterns and food preferences compared with adults. In fact, carbonated water-based flavoured drinks are likely to be the major contributors to the benzoic acid exposure in teenagers because of their high consumption levels of these products. 2 Total diet study conducted by the Food Standards Australia New Zealand in 2005 further revealed that the major contributors to benzoates exposure of teenagers aged 13-18 years were soft drink, orange juice and cordial which accounted for more than 90% of the total benzoates exposure. 3
|
|
5.
|
In view of potential exposure exceeding the ADI established by JECFA particularly in children, JECFA will conduct another assessment after comprehensive information on the levels of benzoic acid used in different types of food in different parts of the world, the results of exposure studies, particularly in children, and other relevant data are gathered. 4
|
|
6.
|
In Hong Kong, past surveillance data were insufficient to provide a clear picture on the levels of benzoic acid used in different prepackaged beverages and the dietary exposure of this chemical in the population. A comprehensive study on dietary exposure to benzoic acid from prepackaged beverages is therefore needed to evaluate the local situation.
|
|
7.
|
Results obtained in this assessment would provide scientific information for prioritising resources in food surveillance activities and form a basis for standard review for benzoic acid in food.
|
Hazard Identification
|
8.
|
Benzoic acid (C 7H 6O 2) has been used as an antimicrobial additive in food for many years. Its activity is primarily against yeasts and moulds, to a lesser extent against bacteria. Undissociated benzoic acid is a more effective antimicrobial agent for preservation; however, its alkali salt form is normally used because of the low solubility of the free acid. 5
|
|
|
9.
|
The o ptimum pH for microbial inhibition by benzoic acid ranges from 2.5 to 4.0. Therefore, b enzoic acid is used for preserving food with high acidity such as soft drinks, fruit juices, salad cream, pickles, tomato sauce and yoghurt. 6 Benzoic acid can also be found naturally in some food of plant and animal origin such as cranberries, prunes, greengage plums, cinnamons, cloves as well as milk products. 7 In general, the naturally occurring benzoic acid in food does not exceed the concentration of 40 mg/kg. 5
|
|
|
10.
|
Apart from food and beverages, benzoic acid is also used in cosmetics, pharmaceuticals, herbicides, flavours and antiseptic ointments for the treatment of fungal infections of the skin etc. 8
|
|
|
11.
|
|
Benzoic acid is most often used as raw material in chemicals production such as phenols, caprolactams, glycol dibenzoates, sodium and potassium benzoates and other benzoic acid derivatives. Benzoic acid can also be detected in car exhaust gases, cigarettes, phytochemically degraded benzoic acid esters in fragrance ingredients, wastewater from wood production industries, foundry waste leachates and ashes from municipal incinerators. 5
|
Hazard Characterisation
|
12.
|
JECFA has evaluated benzoic acid and its salts several times. 9 Generally speaking, benzoic acid is low in acute and chronic toxicity.
|
Kinetics and metabolism
|
13.
|
After the ingestion of benzoates in human, the acidic condition of stomach moves the equilibrium to undissociated benzoic acid, which is rapidly absorbed in the gastrointestinal tract. Most benzoic acid is then metabolised in the liver by glycine conjugation, resulting in hippuric acid formation. Hippuric acid does not accumulate and is rapidly excreted in urine within 10 to 14 hours; u p to 97% may be excreted in the first 4 hours. 5
|
|
14.
|
As the rate limiting step for hippuric acid formation from benzoic acid involves glycine, the availability of glycine affects the biosynthesis of hippuric acid. Therefore, the intake of benzoic acid may affect any function or metabolism in body involving glycine, which results in a reduction of creatinine, glutamine, urea, and uric acid levels. 5
|
Acute effects
|
15.
|
Based on the available animal data, benzoic acid is low in acute oral toxicity. The acute oral LD 50 (the lethal dose administered that kills half of the experimental animals) of benzoic acid and sodium benzoate in rodents is more than 1940 mg/kg bw (body weight). 5
|
Genotoxicity and carcinogenicity
|
16.
|
The International Agency for Research on Cancer (IARC) has not evaluated the carcinogenicity of benzoates. However, there is no evidence of carcinogenicity for benzoic acid and its salts. Nevertheless, genotoxic tests on sodium benzoate with mammalian cells gave consistently positive results. Positive result was also obtained in an in vivo study (dominant lethal assay in rats). Based on the available evidence, the genotoxic activity of sodium benzoate could not be ruled out entirely. 5
|
Chronic effects
|
17.
|
In a four-generation study, no effects on life span, growth rate, fertility, lactation or organ weight were observed in rats fed a diet containing up to 1% benzoic acid (500 mg/kg bw/day).5 |
Reported effects on human
|
18.
|
In a 5-day human volunteer study ingesting up to 2500 mg/day benzoic acid and sodium benzoate, signs of discomfort and malaise including nausea, headache, weakness, burning and irritation of oesophagus were reported. However, symptoms including urticaria, asthma, rhinitis, or anaphylactic shock which appeared shortly after low doses exposure to benzoic acid and sodium benzoate were also reported as both substances are known to cause non-immunological contact reactions (pseudoallergy) in sensitive persons.5 |
Formation of benzene from benzoates and ascorbic acid (vitamin C)
|
19.
|
Benzene forms, at very low level (ppb level), in some beverages containing both benzoates and ascorbic acid. Exposure to heat and light further stimulate the reaction. 10
|
|
20.
|
In 2006, the US Food and Drug Administration (FDA) and the UK Food Standard Agency (FSA) conducted surveys on benzene in soft drinks available in their countries. Results showed that benzene was found in a small range of beverages which contained either added or naturally occurring benzoates and ascorbic acid. About 5.0% of the samples taken by FDA and 2.6% of the samples taken by FSA were detected with a benzene level higher than 5 ppb and 10 ppb (parts per billion) respectively. Affected products had subsequently either been reformulated by the manufacturers or removed from sale. In follow-up samplings, all reformulated products were found to have a benzene level less than 1 ppb. Both surveys concluded that the benzene levels found in soft drinks and other beverages to date do not pose a safety concern for consumers. 10,11 In Hong Kong, out of 84 non-alcoholic beverages sampled from January 2005 to April 2007, none of them were detected with a benzene concentration greater than 5 ppb.
|
|
21.
|
Up till now, Codex has not set any standards for benzene in foods or drinks. The WHO has set a guideline value for benzene of 0.01 mg/l (10 ppb) in drinking water.12 |
Safety reference value
|
22.
|
JECFA re-evaluated and maintained an ADI of 0 - 5 mg/kg bw for benzoic acid and its salts (calcium, potassium and sodium), benzaldehyde, benzyl acetate, benzyl alcohol and benzyl benzoate, expressed as benzoic acid equivalents, in 1996.13 |
Exposure Assessment
Scope of study
|
23.
|
To estimate the dietary exposure to benzoic acid, this study focused on the major contributor – prepackaged beverages. Five major beverage groups were included in this study, namely (i) soft drink (both diet/light and regular types), (ii) fruit juice, (iii) soy milk, (iv) Chinese tea and (v) coffee/tea. |
Methodology
Food consumption data
|
24.
|
The food consumption data in this report was extracted from the Food Consumption Survey among local secondary school students conducted in 2000 by the Food and Environmental Hygiene Department 14. In the survey, a stratified three-stage sampling plan was used, with a sampling frame of 472 secondary schools and more than 380,000 students, covering almost all the local secondary schools. A total of 967 students from 27 schools participated in the survey yielding a response rate of 77% at the school level and 96% at the student level. The mean weight of the participants was 52.0 kg. 14
|
|
25.
|
Consumption of various types of beverages was obtained in the study. However, only the consumption of juice drink was obtained and that of fruit juice was not available. Despite general standards including product definition for fruit juices and nectars have been laid down in Codex, the general public may not be aware of the differences between fruit juice and juice drink. Part of the juice drink consumption data obtained in Food Consumption Survey 2000 was believed to be fruit juice. |
|
26.
|
As the consumption ratio between fruit juice and juice drink is not available in Hong Kong , for the purpose of this study it is assumed that the consumption data of juice drink referred to that of fruit juice. |
Sampling plan
|
27.
|
Different brands of prepackaged beverages were obtained from the local market during August and September 2006. In this study, seasonal sampling was considered not necessary as benzoic acid concentrations were unlikely to vary between seasons in prepackaged beverages. |
Laboratory analysis
|
28.
|
Samples were tested for benzoic acid by the Food Research Laboratory of the Centre for Food Safety. The in-house method was developed with reference to the AOAC Official Method 994.11 Benzoic Acid and Orange Juice – Liquid Chromatographic Method and the analytical methods used in the Survey of Benzoates and Sorbates in Soft Drinks by the UK FSA and the study entitled Simultaneous Determination of Preservatives (Benzoic Acid, Sorbic Acid, Methylparaben and Propylparaben) in Foodstuffs Using High-performance Liquid Chromatography. 2,15
|
|
29.
|
A representative amount of sample was taken and extracted by an acidified alcoholic solution. Benzoic acid was subsequently separated by High Performance Liquid Chromatography using a reversed phase C-18 column and the amount of substance was quantified at 235 nm. The limit of detection (LOD) for benzoic acid was 5 mg/kg. |
|
30.
|
In this study, it was assumed that any benzoic acid detected was intentionally added to provide its technological function. When the analytical result was less than the LOD i.e. <5 mg/kg, it was assumed that benzoic acid had not been added and its concentration is supposed to be zero when calculating the average concentration of benzoic acid in each beverage group. |
Dietary exposure
|
31.
|
The dietary exposures to benzoic acid from prepackaged beverages of average and high consumers were calculated based on the individual food consumption data, body weight and the analytical data of samples. A total of 856 valid food consumption data, 467 boys and 389 girls, were taken into account in this study. Individual benzoic acid exposure per each beverage group was obtained by combining the individual consumption data with the mean concentration of benzoic acid in each group and adjusted with the individual body weight. The dietary benzoic acid exposure of an individual from prepackaged beverages was obtained by summing the exposures from the five beverage groups. The mean and 95th percentile exposure levels represented the benzoic acid exposures for average and high consumers of secondary school students from prepackaged beverages respectively. |
|
32.
|
The benzoic acid exposure levels from prepackaged beverages were compared with the ADI established by JECFA. |
Results of exposure assessment
Food consumption data
|
33.
|
Food consumption data for the five beverage groups are given in Table 1. |
Table 1. The average consumption pattern (prepackaged beverages) for secondary school students (ml/day)
|
Beverage |
11-14 (years) |
15-19 (years) |
11-19 (years) |
||
|---|---|---|---|---|---|
|
Boys |
Girls |
Boys |
Girls |
Overall |
|
|
Soft drink |
217 |
107 |
239 |
89 |
169 (33.1%) |
|
Fruit juice |
136 |
112 |
131 |
114 |
124 (24.3%) |
|
Soy milk |
90 |
65 |
97 |
67 |
81 (15.8%) |
|
Chinese tea |
81 |
103 |
105 |
87 |
94 (18.4%) |
|
Coffee/tea |
37 |
28 |
69 |
40 |
43 (8.4%) |
|
Total consumption |
561 |
415 |
641 |
397 |
511 |
Concentrations of benzoic acid in prepackaged beverages
|
34.
|
A total of 211 prepackaged beverages were sampled for benzoic acid analysis. Results are summarised in Table 2. No benzoic acid was detected in soy milk, Chinese tea or coffee/tea samples. Out of 211 samples tested, 36 (17.1%) contained benzoic acid. |
|
35.
|
Benzoic acid was detected in 52.5% of the soft drink samples. The highest detected benzoic acid concentration in soft drink was 410 mg/kg, which exceeded the legal limit of 160 mg/kg in soft drinks for consumption without dilution. Although only 7.7% of the fruit juice samples contained benzoic acid, one of them was found to contain 580 mg/kg benzoic acid, which was the highest detected concentration in this study. However, this level still fell below the legal limit of 800 mg/kg in fruit juices. |
Table 2. Benzoic acid concentrations in prepackaged beverages
|
Beverage |
No. of analyses |
No. of positive samples |
Average concentration (mg/kg) |
Concentration |
|---|---|---|---|---|
|
Soft drink |
59 |
31 (52.5%) |
78.8 |
n/d - 410 |
|
Fruit juice |
65 |
5 (7.7%) |
16.0 |
n/d - 580 |
|
Soy milk |
29 |
0 (0%) |
0 |
n/d |
|
Chinese tea |
31 |
0 (0%) |
0 |
n/d |
|
Coffee/tea |
27 |
0 (0%) |
0 |
n/d |
|
Total |
211 |
36 (17.1%) |
27.0 |
n/d - 580 |
Not detected (n/d) means results are less than the LOD (LOD = 5 mg/kg)
|
36.
|
The concentrations of benzoic acid in most positive samples (83.3%) ranged from 100 to 199 mg/kg. Three samples were found with benzoic acid level <100 mg/kg but exceeding the LOD and another three samples were found to contain ≥ 300 mg/kg benzoic acid (Figure 1). |
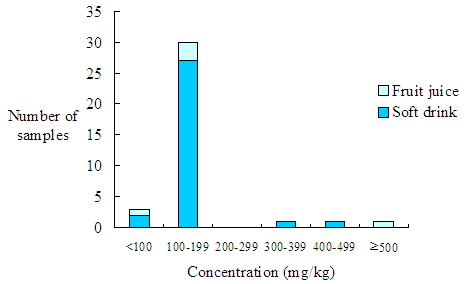
Figure 1. Distribution of benzoic acid concentration in benzoic acid-containing beverages
Levels of dietary exposure
|
37.
|
In this study, all tested samples were water-based and their default densities were 1 g/cm3. Percentage contributions of the major contributing beverages to mean benzoic acid dietary exposure for secondary school students are in Figure 2. Soft drinks contributed to about 80% of the benzoic acid exposures from prepackaged beverages of secondary school students. |
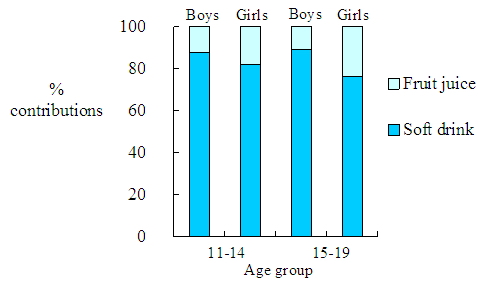
Figure 2. Percentage contributions of the major contributing beverages to mean benzoic acid dietary exposure for secondary school students
Average secondary school students – by age and sex categories
|
38.
|
The average dietary exposure of secondary school students to benzoic acid from prepackaged beverages was 0.31 mg/kg bw/day, ranging from 0.17 to 0.41 mg/kg bw/day in different age and sex categories (Table 3). The dietary exposure to benzoic acid depends not only on the consumption pattern but also on the body weight. Generally, secondary school girls are less exposed to benzoic acid from prepackaged beverages than boys. Similarly, the older populations with higher body weight are less exposed to benzoic acid than the younger populations with lower body weight. |
Table 3. Average dietary exposure to benzoic acid for secondary school students from prepackaged beverages by age and sex categories (mg/kg bw/day)
|
Age |
Boys |
Girls |
|---|---|---|
|
11-14 |
0.41 |
0.22 |
|
15-19 |
0.36 |
0.17 |
High consumers – by age and sex categories
|
39.
|
Further analysis was undertaken to estimate the risk that high consumers might be exposed to. The 95th percentile exposure level of secondary school students was used to represent the dietary exposure to benzoic acid from prepackaged beverages for a high consumer. The dietary benzoic acid exposure from prepackaged beverages for hi gh consumers was 0.97 mg/kg bw/day, ranging from 0.58 to 1.30 mg/kg bw/day in different age and sex categories (Table 4). |
Table 4. Dietary exposure to benzoic acid for high consumers (95th percentile) from prepackaged beverages by age and sex categories (mg/kg bw/day)
|
Age |
Boys |
Girls |
|---|---|---|
|
11-14 |
1.30 |
0.63 |
|
15-19 |
1.11 |
0.58 |
Risk Characterisation
Dietary exposure
|
40.
|
The average dietary exposure to benzoic acid for secondary school students was estimated to be 0.31 mg/kg bw/day whilst that for the high consumers was 0.97 mg/kg bw/day. The exposure amounted to 6.1% (ranging from 3.3% to 8.2% in different age and sex categories) and 19.3% (ranging from 11.7% to 26.0% in different age and sex categories) of the ADI respectively (Figure 3). |
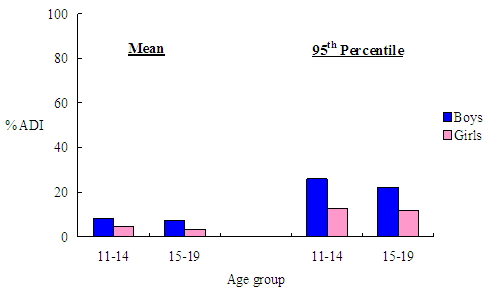
Figure 3. Dietary benzoic acid exposure as a percentage of ADI for average and high consumers (95th percentile) from prepackaged beverages
|
41.
|
Exposures to benzoic acid for both average secondary school students and high consumers fell well below the ADI established by JECFA. The dietary exposure estimates therefore suggested the average and high consumers of secondary school students were unlikely to experience major adverse health effects of benzoic acid. |
Specific brand loyalty
|
42.
|
In this study, the benzoic acid exposure was calculated with the average benzoic acid concentration detected in each beverage group. However, in reality, brand loyalty in which consumer preferred to have a specific brand of product is common. To address this issue, a worst-case scenario was chosen, where the benzoic acid exposure was calculated using the same food consumption data but the highest benzoic acid concentration detected in this study under the fruit juice group. |
|
43.
|
Based on the assumptions that students consumed the beverage with benzoic acid concentration at 580 mg/kg under the fruit juice group specifically and had no special preferences in other beverage groups, the benzoic acid exposure of an average secondary school student would be 1.71 mg/kg bw/day (ranging from 1.43 to 2.07 mg/kg bw/day in different age and sex categories) while that of high consumers would be 4.78 mg/kg bw/day (ranging from 3.77 to 6.38 mg/kg bw/day in different age and sex categories). The exposure amounted to 34.3% of the ADI for average secondary school students and 95.7% of the ADI for the high consumers respectively. Generally, under the worst-case scenario, exposure to benzoic acid for average secondary school students still fell below the ADI, however, for that of high consumers (boys aged 11-14) exceeded the ADI by 28.0% (Figure 4). |
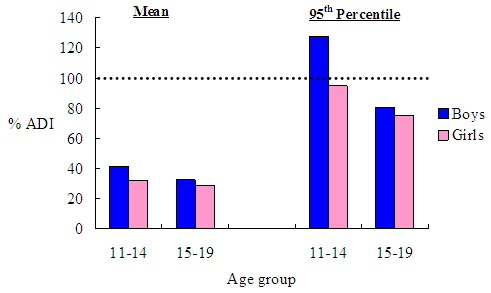
Figure 4. Dietary benzoic acid exposure as a percentage of ADI for average and high consumers (95th percentile) with specific brand loyalty towards the fruit juice with benzoic acid level at 580 mg/kg
Risk estimate
|
44.
|
Based on the findings from Food Standards Australia New Zealand, more than 90% of the total benzoates exposure in teenagers aged 13-18 years were from soft drinks, orange juice and cordial. 3 In other countries like France , the United Kingdom and the United Sates, soft drinks also contributed most to the benzoic acid intake. 3 Therefore, results of this study indicated there is no significant adverse health effect of benzoic acid from normal dietary consumption in local secondary school students.
|
|
45.
|
As indicated in the worst-case scenario where consumers had specific brand loyalty towards the fruit juice sample with the highest benzoic acid level detected in this study; the general exposure to benzoic acid from prepackaged beverages in high consumers (boys aged 11-14 years) would exceed the ADI. However, this finding might be overestimated by some degree.
|
|
46.
|
For the purpose of this study, it is assumed that the juice drink consumption data obtained in Food Consumption Survey 2000 referred to that of fruit juice. Nevertheless, some of the consumption volume should belong to the category of juice drink (a type of soft drink). Assuming the juice drink consumption volume as that of fruit juice would result in overestimation. |
|
47.
|
The exposure can be better estimated if the consumption ratio between fruit juice and juice drink is available. To take into account of this limitation, an exposure range has been estimated using different consumption ratios by estimating benzoic acid exposure of secondary school students in worst case scenario at the two extremes (Table 5). The lower range was calculated b y categorising the juice drink consumption data into that of soft drink while the upper range was calculated by categorising the juice drink consumption data into that of fruit juice. |
Table 5. Dietary benzoic acid exposure range as a percentage of ADI for average and high consumers (95th percentile) in worst-case scenario
|
|
Average (% ADI) |
High consumers (%ADI) |
|---|---|---|
|
11-14 boys |
11.9 - 41.4 |
38.4 - 127.5 |
|
11-14 girls |
7.5 - 32.0 |
20.0 - 94.8 |
|
15-19 boys |
10.1 - 32.5 |
27.5 - 80.7 |
|
15-19 girls |
6.4 - 28.6 |
19.0 - 75.4 |
|
48.
|
Based on the best available information, the dietary exposures to benzoic acid from prepackaged non-alcoholic beverages of secondary school student were likely to fall in between the ranges of the above estimates under the worst-case scenario, which were generally below the ADI for benzoic acid.
|
|
49.
|
ADI for benzoic acid was established based on the no-observed-effect-level (NOEL) of a long-term exposure study in rats, where no adverse effects were observed even at the highest dose tested (500 mg/ kg bw/day). A safety factor of 100 was applied to the NOEL taking species and individual human differences into consideration. Possibility for high consumers with specific brand loyalty towards items with high benzoic acid concentration to exceed the ADI is a concern which effectively reduces the margin of safety provided by the ADI.
|
|
50.
|
However, ADI is an estimated amount of a substance that can be ingested daily over a lifetime without appreciable health risk. An exposure above the ADI does not automatically mean that health is at risk. Transient excursion above the ADI would have no health consequences provided that the average exposure does not continuously exceed the ADI which emphasises on a lifetime exposure. |
|
51.
|
Average and high consumers of secondary school students with a normal balanced diet were not likely to experience any undesirable effects of benzoic acid from the intake of prepackaged beverages. |
Major contributor of benzoic acid exposure
|
52.
|
In this study, the major contributor to benzoic acid of secondary school students was soft drink, which contributed to about 80% of the total exposure from prepackaged beverages. The high benzoic acid exposure of secondary school students from soft drink was because this group of drink had a relatively high average benzoic acid concentration (78.8 mg/kg) when compared with fruit juice (16.0 mg/kg) as well as a considerably large amount of consumption i.e. >30%. |
Violation of the Preservatives in Food Regulations
|
53.
|
As stipulated in the Preservatives in Food Regulations, First Schedule Part I, the maximum permitted benzoic acid concentration in soft drinks is 160 mg/kg. Two soft drinks, with detected concentration at 310 mg/kg and 410 mg/kg respectively, exceeded the benzoic acid limit prescribed by law. |
|
54.
|
The purpose of this study was to estimate the dietary exposure to benzoic acid rather than law enforcement. However, in response to the non-compliance, the Centre for Food Safety (CFS) issued warning letters to relevant parties and all the affected products were taken off sale and surrendered for disposal voluntarily. Further actions would be taken if the products are found to be back to the shelves. |
International scenario
|
55.
|
Results from different studies on dietary exposure to benzoic acid conducted during 2000-2007 are summarised in Table 6. |
Table 6. A comparison of average dietary exposure to benzoic acid (mg/kg bw/day)
|
Locations |
Average dietary exposure to benzoic acid |
|---|---|
|
Australia3 |
0.96* |
|
France16 |
0.40§ |
|
Korea17 |
0.009-0.025‡ |
|
Hong Kong |
0.31† |
|
United Kingdom16 |
0.50†† |
|
*
|
The average daily dietary exposure to benzoic acid in consumers aged 13-18 years
|
|
§
|
The estimated benzoates intake in average consumers aged 10-17 years
|
|
‡
|
The estimated daily exposure to benzoic acid for average consumers of different age groups
|
|
†
|
The average daily dietary exposure is extracted from our current study, which estimated only from the prepackaged beverages exposure of secondary school students aged 11-19 years
|
|
††
|
The estimated benzoates intake in average children
|
|
56.
|
It can be seen that dietary exposure to benzoic acid estimated in our study is in similar order of magnitude when compared to exposure estimates obtained from overseas studies, except the one conducted in Korea . Such difference might due to the absence of benzoic acid in carbonated beverages as well as exclusion of juice from the study. This comparison gives a general idea of the local situation in the international context, but it should be noted that direct comparison of data between different studies has to be done with caution due to the differences in research methodology, food group categorisation, consumption data collection methods, methods of benzoic acid analysis and the time when the studies were carried out. |
Limitations of the Exposure Assessment Study
|
57.
|
In this study, the exposure assessment based only on the consumption of prepackaged non-alcoholic beverages within the five beverage groups, benzoic acid exposures from other types of drinks such as yoghurt drinks as well as food or sauces were not taken into account. The percentage contribution of prepackaged beverages to the total dietary benzoic acid exposure in the local population is also unknown. Furthermore, exact consumption figures separately for juice and juice drink are not available.
|
|
58.
|
The methodology for collecting food consumption data may affect the accuracy of the estimates on dietary exposure. In the Food Consumption Survey, the food consumption pattern of secondary school students was collected using a food frequency questionnaire. Although the questionnaire was very comprehensive, it was not possible to cover every beverage item, some of which might contain benzoic acid. Furthermore, only consumption pattern for secondary school students is available, which may not reflect the situation of the whole population. |
|
59.
|
Although 211 prepackaged beverages were sampled in this study, increasing the number of samples for laboratory analysis could provide a more precise estimate of the average benzoic acid concentration in a particular beverage group. However, the number of samples taken have to be balanced with the available resources and number of beverage items to be included. In order to maximise the number of different beverages analysed, there was no duplication in sampling of products. |
Conclusion and recommendation
|
60.
|
Out of 211 samples tested, 36 (17.1%) contained benzoic acid. No soy milk, Chinese tea or coffee/tea samples were detected with benzoic acid. One of the fruit juice samples was found to contain the highest detected benzoic acid level (580 mg/kg) in this study.
|
|
61.
|
The dietary exposures to benzoic acid for average and high consumers of the secondary school students from prepackaged beverages were 0.31 and 0.97 mg/kg bw/day respectively. Both exposure levels fell well below the ADI established by JECFA. It could be concluded that both the average and high consumers of the secondary school students were not likely to experience any adverse effects of benzoic acid from the exposure of prepackaged beverages.
|
|
62.
|
In the worst-case scenario where consumers had specific brand loyalty towards the fruit juice sample with the highest benzoic acid level detected in this study; the general exposure to benzoic acid from prepackaged beverages in high consumers (boys aged 11-14 years) would exceed the ADI. Although this exposure might have been overestimated to a certain degree, it raised a potential public health concern for secondary school students consuming beverages containing high levels of benzoic acid. Further studies were recommended to examine this issue further.
|
|
63.
|
Members of the trade should comply with the Public Health and Municipal Services Ordinance and its subsidiary legislations, including only using appropriate amount of benzoic acid and its sodium, potassium and calcium salts in specific food products prescribed by law as well as correctly labelling them (exact name or its identification number under the International Numbering System (INS)) in the ingredient list together with the technological function. They are also advised to observe good manufacturing practices to limit the use of food additives i.e. benzoic acid in food and drinks to the lowest possible level necessary to accomplish its desired effect. |
|
64.
|
The public is advised to maintain a varied and balanced diet so as to avoid excessive exposure of benzoic acid and other food additives from a small range of food items. For benzoic acid sensitive individuals, they are advised to read food labels carefully before purchasing or consuming prepackaged food to see whether the food contains benzoic acid and seek advice from medical professionals when necessary. |
References
1 Piper, P.W. Yeast Superoxide Dismutase Mutants Reveal a Pro-oxidant Action of Weak Organic Acid Food Preservatives. Free Radical Biology and Medicine. 1999; 27: 1219-1227.
2 Food Standard Agency (FSA). Survey of Benzoates and Sorbates in Soft Drinks - No. 84/05. FSA; November 2005. Available from URL: http://www.food.gov.uk/multimedia/pdfs/fsis8405.pdf#page=20
3 Food Standards Australia New Zealand (FSANZ). The 21st Australian Total Diet Study - a total diet study of sulphites, benzoates and sorbates. Canberra : FSANZ; August 2005. Available from URL: http://www.foodstandards.gov.au/newsroom/publications/21staustraliantotald2963.cfm
4 Codex Alimentarius Commission (CAC). Joint FAO /WHO Food Standards Programme - Report of the Twenty-seventh Session. ALINORM 04/27/41 . CAC: Geneva ; 2004. Available from URL: ftp://ftp.fao.org/docrep/fao/007/y5549e/y5549e00.pdf
6 Belitz, H.-D.; Grosch, W. Food Chemistry. 2 nd edition. Springer. New York ; 1999.
7 Reddish, G.F. Antiseptics, Disinfectants, Fungicides and Sterilization. 2nd edition. Lea and Febiger. Philadelphia ; 1957.
8 CHEMINFO. Chemical Profiles Created by Canadian Centre for Occupational Health and Safety (CCOHS). Ontario : CCOHS; 1998. Available from URL: http://www.intox.org/databank/documents/chemical/benzoic/cie374.htm
9 The Joint FAO/WHO Expert Committee on Food Additives (JECFA). Summary of Evaluations Performed by JECFA – Benzoic acid. Rome : FAO; May 2005. Available from URL: http://www.inchem.org/documents/jecfa/jeceval/jec_184.htm
10 FDA. Data on Benzene in Soft Drinks and Other Beverages. FDA: Maryland ; May 2006. Available from URL: http://www.cfsan.fda.gov/~dms/benzdata.html
11 FSA. Survey of Benzene of Soft Drinks – No. 06/06. FSA; March 2006. Available from URL: http://www.food.gov.uk/multimedia/pdfs/fsis0606.pdf
12 WHO. Guidelines for Drinking-water Quality. Geneva : WHO; 2003. Available from URL: http://www.who.int/water_sanitation_health/dwq/chemicals/benzenesum.pdf
13 WHO. Toxicological Evaluation of Certain Food Additives. Prepared by the 46th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series 37. Geneva : WHO; 1996. Available from URL: http://www.inchem.org/documents/jecfa/jecmono/v37je05.htm
14 FEHD. Food Consumption Survey 2000. Hong Kong : FEHD; 2001.
15 Saada, B.; Baria, M.F; Saleha, M.I.; Ahmadb, K.; Talib, M. K. M. Simultaneous Determination of Preservatives (Benzoic acid, Sorbic acid, Methylparaben and Propylparaben) in Foodstuffs Using High-performance Liquid Chromatography. Journal of Chromatography A. 2005; 1073: 393-397.
16 International Programme on Chemical Safety. Evaluation of National Assessments of Intake of Benzoates. WHO Food Additives Series No. 42. Geneva : WHO; 1999. Available from URL: http://www.inchem.org/documents/jecfa/jecmono/v042je22.htm
17 Yoon, H.J.; Cho, Y.H.; Juyeon, P.; Lee, C.H.; Park, S.K.; Cho, Y.J.; Han, K.W.; Lee, J.O.; Lee, C.W. Assessment of Estimated Daily Intakes of Benzoates for Average and High Consumers in Korea. Food Additives and Contaminants. 2003; 20: 127-135.