
Food Safety Focus (170th Issue, September 2020) – Incident in Focus
Calcium Oxalate – the Stinging Crystals in Plants
Reported by Dr. John LUM, Scientific Officer,
Risk Assessment Section, Centre for Food Safety
In August 2020, the Centre for Health Protection of the Department of Health investigated a suspected food poisoning case related to calcium oxalate raphides. In fact, among plant poisoning cases referred to the Hospital Authority Toxicology Reference Laboratory from 2003 to 2017, around one-fourth are related to calcium oxalate raphides. So, what is calcium oxalate and why does it hurt?
Ouch! Why Does Calcium Oxalate Hurt?
Many plants contain oxalates. Some oxalates, such as sodium and potassium oxalates, are water soluble, and others form insoluble crystals, such as calcium and magnesium oxalates. Calcium oxalates crystals have been found in more than 200 plant families and in almost all types of tissues of these plants, including leaf, stem, root and even anther.
Calcium oxalate crystals are found in several shapes in plants, including needle-shaped 'raphides', pencil-shaped 'styloids', block-shaped 'crystal sand' and rosette-shaped 'druses'. Among them, the needle-shaped raphides catch the most clinical attention.
The sharp needle-shaped raphides are packed in bundles within plant cells. Damage to the plant cells during chewing causes water to enter and swell up the cells. This stimulates the forceful propulsion of the sharp raphides into the surrounding environment, stabbing the sensitive tissues of the tongue, gums and throat and resulting in tissue injuries in the oral cavity.

Figure 1a: Microscopic examination of calcium oxalate raphides in the stem and corm of wild taro.
(Courtesy of the Hospital Authority Toxicology Reference Laboratory)
Some Calcium Oxalate Crystals Are Less Irritating
It is believed that the needle-shaped calcium oxalate is the most irritating, while other shapes are less likely to inflict damage. The calcium oxalate crystals of wild taro are needle-shaped, compared with those block-shaped ones found in spinach. Therefore, although calcium oxalate crystals are present in both wild taro and spinach, only the crystals in wild taro can cause agonising irritation, whereas those in spinach have little effect.
Besides the shape of the calcium oxalate crystals which mainly contributes to the irritating effect, other factors are also involved. For example, the presence of enzymes in some plant cells that facilitate the breakdown of proteins may further potentiate the irritation through triggering an inflammatory reaction. Some raphides even have grooves which hold these enzymes. This is the reason why the acrid taste of raw edible taro is substantially reduced after cooking, as cooking destroys the enzymes. However, cooking cannot remove the poisoning effect of wild taro and it should not be consumed.
Presentation of Calcium Oxalate Poisoning
The symptoms of calcium oxalate poisoning are mainly localised, causing oral and sometimes upper airway irritation. Systemic absorption of the chemical is nevertheless rare, as the instantaneous pain and swelling of the mouth caused by raphides usually stop the affected person from further ingesting a significant quantity of the plant. Rapid and immediate in onset, these symptoms are mild in most cases, usually resolving within a short period of time.
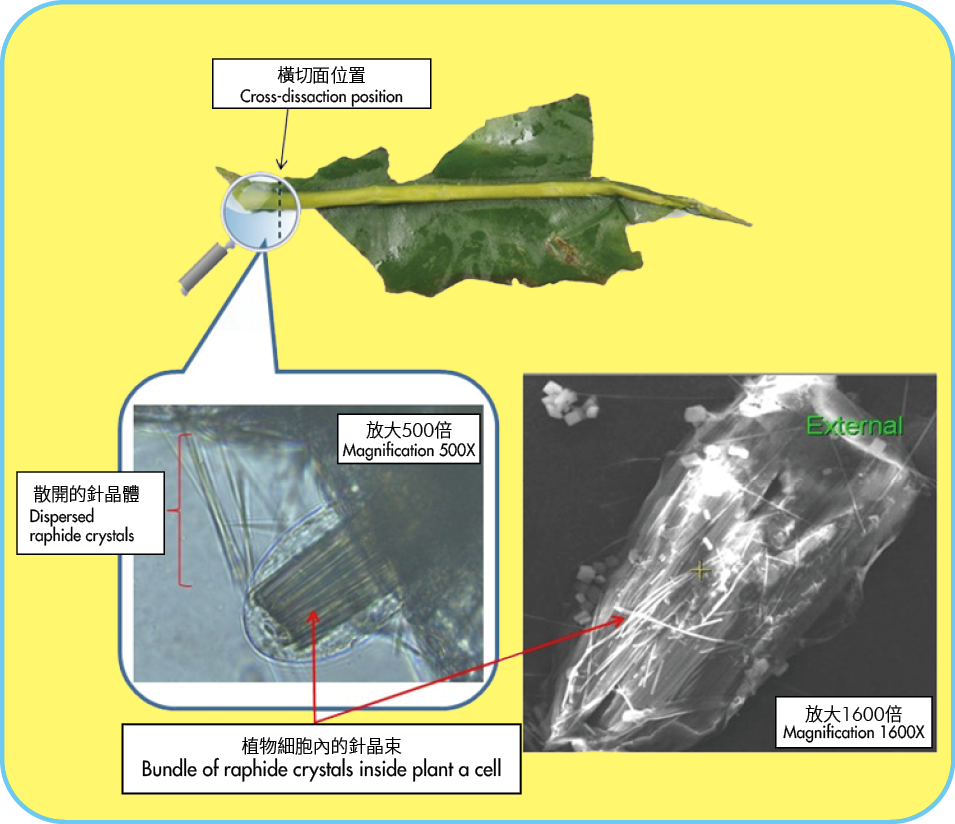
Figure 1b: Microscopic examination of a leaf sample from a food complaint case, showing the presence of calcium oxalate raphides in the sample.
(Courtesy of the Government Laboratory)
Suspected Food Items Related to Calcium Oxalate Raphide Poisoning
Locally, previous food poisoning cases of calcium oxalate raphides mainly involved the consumption of wild taro, which is known to contain calcium oxalate raphides. Other cases involved different types of vegetables, such as water spinach, Chinese white cabbage, Chinese flowering cabbage and watercress. These vegetables are not supposed to contain calcium oxalate raphides, and it is postulated that the vegetables might have been mixed with small amount of raphide-containing plants and consumed. In some of these incidents, leaves of wild taro may have been used to wrap or cover vegetables during transportation and processing.
Prevention
Calcium oxalate crystals cannot be dissolved in water and are heat resistant. Therefore, washing and cooking cannot reliably remove calcium oxalates from the toxic plants. The most effective way to prevent calcium oxalate poisoning is not to consume plants that are not known to be edible, and to avoid their contamination to edible vegetables.

Figure 2: Leaf of wild taro, which is known to contain calcium oxalate raphides. Broken parts of the leaf may contaminate vegetables during transportation or processing.
Key Points to Note
- Some plants (e.g. wild taro) are known to contain needle-shaped calcium oxalate raphides and should not be consumed.
- Upon ingestion, calcium oxalate raphides cause tissue irritation and pain in the mouth.
- Washing and cooking cannot reliably remove calcium oxalate raphides from plants.
Advice to Consumers
- Never pick and eat wild plants such as wild taro, as they could be poisonous.
- Buy vegetables from reliable suppliers.
- Remove any unidentified plants and objects mixed with vegetables.
- In case of feeling unwell after food consumption, seek immediate medical attention and bring along the food remnant, if any.
Advice to the Trade
- Do not use leaves of wild taro to cover or wrap vegetables during transportation and processing, as they may cross-contaminate the vegetables.
- Care should be taken to prevent the mixing of vegetables with other inedible plants.

