Risk Assessment Studies
Report No. 26
Chemical Hazard Evaluation
DIETARY EXPOSURE TO ANTIMONY
OF
SECONDARY SCHOOL STUDENTS
January 2007
Centre for Food Safety
Food and Environmental Hygiene Department
The Government of the Hong Kong Special Administrative Region
Correspondence:
Risk Assessment Section
Centre for Food Safety
Food and Environmental Hygiene Department
43/F, Queensway Government Offices,
66 Queensway, Hong Kong.
Email: enquiries@fehd.gov.hk
Table of Contents
- Abstract
- Objectives
- Introduction
- Hazard Identification
- Hazard Characterisation
- Exposure Assessment
- Risk Characterisation
- Limitations of the Exposure Assessment Study
- Conclusion and Recommendation
- References
- Annex: Distribution Curves of Antimony Concentrations in Six Food Groups
ABSTRACT
This study estimated the dietary exposure to antimony of secondary school students in Hong Kong and assessed the associated health risks. Dietary exposure to antimony was estimated using the local food consumption data obtained from secondary school students in 2000 and the concentrations of antimony in food samples taken from the local market. Laboratory analysis for antimony was conducted by the Food Research Laboratory of the Centre for Food Safety.
The dietary exposures to antimony for average and high consumers of the secondary school students were 0.036 and 0.081 μg/kg bw/day respectively. Both levels fell well below the safety reference value (i.e. the Tolerable Daily Intake (TDI)) of 6 μg/kg bw/day established by the World Health Organization and amount to less than 2% of this safety reference value. It could be concluded that both the average and high consumers of the secondary school students were unlikely to experience major toxicological effects of antimony.
The results also showed that the food group "cereal and cereal products", particularly rice, was the main dietary source of antimony.
Because of the ubiquitous nature of antimony, low levels of antimony in foods might be unavoidable. Food trade is recommended to observe good agricultural and manufacturing practices to minimise antimony contaminations in food. Members of the public are advised to maintain a balanced diet so as to avoid excessive exposure from a small range of food items.
OBJECTIVES
This study aims to estimate the dietary exposure to antimony of secondary school students in Hong Kong and to assess the associated health risk.
INTRODUCTION
2. The presence of metals in food, including antimony, arsenic, cadmium, chromium, lead, mercury and tin, is regulated under the Food Adulteration (Metallic Contamination) Regulations (Cap. 132 sub. leg.). Among which, four heavy metals, namely arsenic, cadmium, mercury and lead, are of particular concern in terms of food safety and public health. The Food and Environmental Hygiene Department therefore conducted studies on dietary exposure to arsenic, cadmium and mercury of secondary school students in 2002, a follow-up study on mercury and methylmercury in 2004 as well as a study on lead in 2005 respectively.
3. Available data 1,2,3 from overseas studies indicated that exposure to antimony through diet is relatively low when compared with the safety reference value established by the World Health Organization (WHO). However, apart from our local legislation, we could not find any safety standard with respect to antimony in food available in the international arena and in various places including Mainland China, the United States, Canada, member countries in the European Union, Australia and New Zealand. However, in view of lacking local data on dietary exposure to antimony, the associated health risk to the local population is not clear. A study on dietary exposure to antimony is therefore needed to examine the situation in the local scene.
4. The result of this assessment would provide scientific information for future risk management, including prioritising resources in food surveillance activities and form a basis for standard review for antimony in food in our local legislation.
HAZARD IDENTIFICATION
5. Antimony has been classified as both a metal and a metalloid. It is a silvery-white, brittle solid present in the earth!|s crust. Antimony has four valence states, with the trivalent form being the most common and stable 4,5,6.
6. Elemental antimony can be used for producing semiconductors, infrared detectors and diodes. Because of its relative inflexibility in nature, it is usually mixed into alloys for further application, e.g., manufacture of lead storage batteries, solder, sheet and pipe metal, bearings, castings and pewter, etc.. Antimony compounds have also been used for treating diseases such as parasitic infection in humans. On the other hand, antimony oxide can be used in fire-retardant formulations for plastics, rubbers, textiles, paper and paints whereas antimony trisulfide is used in the production of explosives, pigments, antimony salts and ruby glass4,6,7.
7. Antimony, usually in the form of antimony trioxide, enters the environment mainly as a result of industrial activities such as coal burning or smelting of antimony-containing ores. Antimony can also be naturally present in the environment via weathering of rocks and runoff from soils. On the other hand, trace amount of antimony in tap water may leach from household piping and non-leaded solders under certain condition, e.g. after 7 days of contact4,6,8.
8. Once released, most antimony ends up in soil with low level in air and water. It is not degradable in the nature and it does not bio-accumulate in living organisms. Therefore, dietary exposure to antimony is expected to be low. Antimony has been reported to be present in food, including fruit, vegetables, meat, freshwater fish, meat and poultry, with higher level being detected in marine food4,6,8.
HAZARD CHARACTERISATION
9. Generally speaking, the toxicity of antimony and its compounds depends on their water solubility and oxidation/valence state, e.g. trivalent antimony is more toxic than pentavalent antimony whereas inorganic forms are more toxic than the organic forms8.
Kinetics and metabolism
10. Studies indicate that the absorption of antimony, even in soluble forms, is relatively low, irrespective of the valence state. An absorption rate of 5% was observed for acute intoxication with potassium antimony tartrate in humans 8.
11. Distribution and excretion of antimony depend on its valence state. Because of its lack of electrical charge, trivalent antimony in the form of antimony trihydroxide can easily pass through cell membranes and have a longer elimination half-time when compared with pentavalent antimony compounds8. On the other hand, trivalent antimony is generally excreted in faeces whereas pentavalent organic antimony is excreted in urine. In humans, majority of antimony absorbed is excreted in urine, others can be eliminated via excretion in faeces, human milk or placental transfer 6.
12. Antimony was shown to accumulate in liver, kidney, bone, lung, spleen and thyroid following ingestion in experimental animals. In humans, trivalent antimony was found to accumulate in liver, thyroid and heart following therapeutic use6. Antimony, nevertheless, is not an essential nutrient for plants and animals8.
Acute effects
13. It was reported that oral median lethal dose (LD50) values for potassium antimony tartrate in experimental animals ranged from 115 to 600 mg/kg body weight (bw) whereas that for antimony trioxide was greater than 20,000 mg/kg bw due to its low water solubility8.
14. Ingesting large amounts of antimony salts can cause irritation of gastrointestinal tract resulting in symptoms including vomiting, abdominal cramps, diarrhoea and cardiac toxicity. In addition, severe myocardial symptoms, convulsions as well as deaths have been observed. The reported oral lethal doses in the form of potassium antimony tartrate was 300 mg and 1200 mg for a child and an adult respectively 7,8.
Genotoxicity and carcinogenicity
15. WHO in 2003 concluded that soluble antimony(III) salts are genotoxic in vitro and in vivo whereas antimony trioxide, due to its low bioavailability, is genotoxic only in some in vitro tests but not in vivo8 .
16. The International Agency for Research on Cancer (IARC) of the WHO has evaluated the carcinogenicity of antimony trioxide and antimony trisulfide. Antimony trioxide has been demonstrated to be cancer causing in rats upon inhalation exposure. In its evaluation in 1989, the IARC considered that there was inadequate evidence for the carcinogenicity of the two compounds in humans, but sufficient evidence for the carcinogenicity of antimony trioxide and limited evidence for the carcinogenicity of antimony trisulfide in experimental animals. The IARC classified antimony trioxide as Group 2B agent (i.e. possibly carcinogenic to humans) and antimony trisulfide as Group 3 agent (i.e. not classifiable as to its carcinogenicity to humans) respectively 7.
17. WHO in 2003 considered that the greatest concern with regard to the carcinogenicity of antimony compounds was related to the inhalation route and there was a lack of appropriate data to evaluate the cancer risks associated with oral antimony exposure. In addition, WHO reported that therapeutic doses of an antimony(V) compound, meglumine antimoniate, has been demonstrated not representing any mutagenic or carcinogenic risks to humans8.
Other chronic effects
18. Chronic occupational exposure to lower doses of antimony compounds may lead to myocardial effects6 . Repeated oral exposure to therapeutic doses of antimony(III) in humans was associated to optic nerve destruction, uveitides and retinal bleeding, generally accompanied by symptoms including headache, coughing, anorexia, troubled sleep and vertigo8. Regarding reproductive and developmental toxicity, there is no conclusive evidence demonstrating such effects6,8.
Safety reference value
19. JECFA has not evaluated the safety of antimony. In developing the Guidelines for Drinking-water Quality, WHO in 2003 established a tolerable daily intake (TDI) of 6μg/kg bw/day for antimony. The TDI was derived from a no-observed-adverse-effect level (NOAEL) of 6.0 mg/kg bw/day based on decreased body weight gain and reduced food and water intake in a subchronic toxicity study in rats, together with a safety factor of 1000. In WHO's assessment, safety factors of 10 were used for inter-species extrapolation, 10 for intra-species variation and another 10 for the use of data from a subchronic study8.
EXPOSURE ASSESSMENT
Scope of study
20. To estimate the dietary exposure to antimony, this study covered six major food groups, namely (i) cereals and cereal products, (ii) vegetables, (iii) fruits, (iv) meat, poultry, egg and their products, (v) seafood, as well as (vi) dairy products. The selection is based on the reported occurrence of antimony in these food groups and the consumption patterns.
Methodology
Food consumption data
21. The food consumption data in this report was extracted from the Food Consumption Survey conducted in local secondary school students in 2000 by the FEHD. In the survey, a stratified three-stage sampling plan was used, with a sampling frame of 472 secondary schools and more than 380,000 students, covering almost all the local secondary schools. A total of 967 students from 27 schools participated in the survey yielding a response rate of 77% at the school level and 96% at the student level. The mean weight of the participated students was 52.0 kg9 .
Sampling plan
22. Food samples were taken from the local market according to the above six food groups. Food items were selected so as to match those in the Food Consumption Survey as well as those with likely occurrence of antimony. Three samples of each food item were taken randomly from different sources for laboratory analysis.
Laboratory analysis
23. Laboratory analysis was conducted by the Food Research Laboratory of the Centre for Food Safety. All food samples were treated and analysed as consumed so as to give a better estimate of antimony concentration to be consumed. The three samples collected were mixed and homogenised to give a composite sample for further freeze-drying. The freeze-dried sample is digested sequentially with concentrated nitric acid, hydrogen peroxide and concentrated hydrochloric acid at 95oC, followed by filtration and then determination of antimony using hydride generation-inductively coupled plasma mass spectrometry (HG-ICP/MS). The limits of detection (LODs) for solid sample and liquid sample were 1 £gg/kg (ppb) and 0.1 £gg/L (ppb) respectively. The adopted LODs are comparable to those used by authorities in the international arena for dietary exposure assessment and are in fact lower than the ones adopted in the Australian Total Diet Survey.
24. When the analytical value was below the LOD, the true value of antimony in the food sample could be anywhere between zero and the LOD. The treatment for these results was particularly important when a large percentage of the analytical results of a particular food group fell below the LOD. While it may not be appropriate to assume a zero concentration for all samples with analytical values below LOD, assigning these non-detects a value of LOD, would, however, grossly overestimate the dietary exposure. In this study, a value of 1/2-LOD was then assigned to all results below LOD. Since the levels of contaminants in food, including antimony, usually follow a log-normal distribution, the approach of assigning a value of 1/2-LOD to all non-detected levels is considered conservative, particularly for food groups in which majority of food items have analytical values below the LOD.
Dietary exposure
25. Daily dietary exposure from an individual food item was obtained by combining the consumption data and the antimony concentration of that food item. Total exposure for each secondary school student was obtained by summing exposures from all food items. The mean and 95th percentile of the daily exposure levels were used to represent the dietary exposure for average and high consumers respectively.
26. The estimated exposure levels were then compared by the TDI established by WHO.
Results of exposure assessment
Food consumption data
27. Food consumption data for the six food groups are given in Table 1.
Table 1: Food Consumption Pattern for Secondary School Students
| Food groups | Mean consumption (g/day) |
|---|---|
| Cereal and cereal products | 478.0 |
| Vegetables | 295.3 |
| Fruits | 309.1 |
| Meat, poultry, egg and their products | 203.7 |
| Seafood | 122.4 |
| Dairy products | 143.2 |
Concentration of antimony in food
28. A total of 300 food samples were taken and combined into 100 composite samples for analysis. The results are summarised in Table 2.
Table 2: Concentration of Antimony in the Six Food Groups
| Food groups | Number of samples | Number of composite samples | % of samples with non-detected amount of antimony | Median concentration* (μg/kg) |
|---|---|---|---|---|
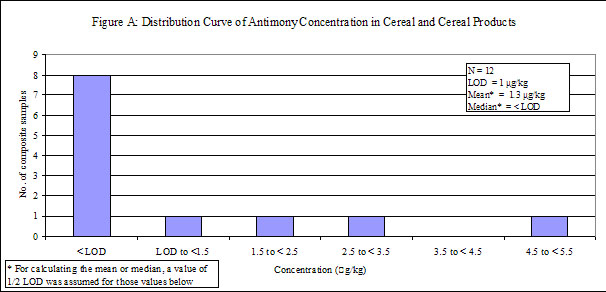
| Cereal and cereal products | 36 | 12 | 67 | < LOD |
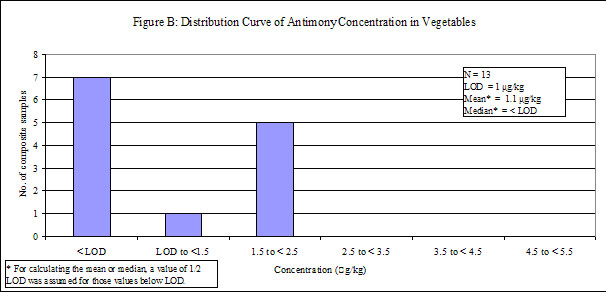
| Vegetables | 39 | 13 | 54 | < LOD |
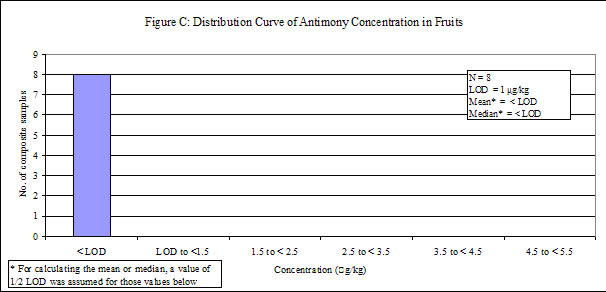
| Fruits | 24 | 8 | 100 | < LOD |
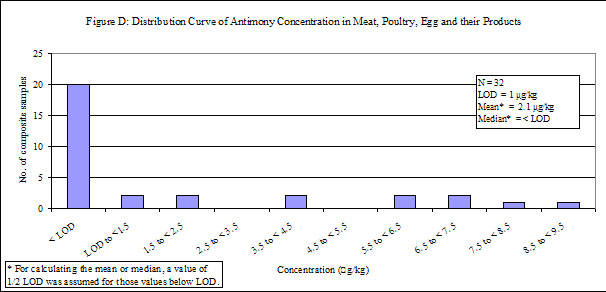
| Meat, poultry, egg and their products | 96 | 32 | 63 | < LOD |
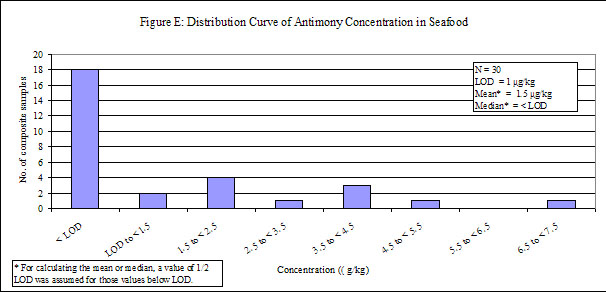
| Seafood | 90 | 30 | 60 | < LOD |
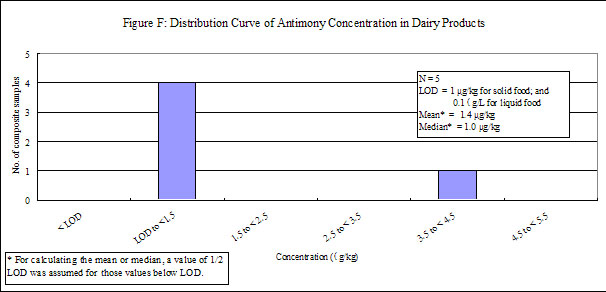
| Dairy products | 15 | 5 | 0 | 1 |
| Total | 300 | 100 | 61 | - |
* A value of 1/2 LOD was assigned, i.e. 0.5 μg/kg for solid food and 0.05 μg/L for liquid food, when the analytical value was <LOD.
29. Antimony was detected in low levels in all of the food groups, except "fruits", in which antimony was not detected. Distribution curves of antimony concentrations in different food groups are presented in the Annex.
Level of dietary exposure
Average secondary school students
30. The dietary exposure to antimony for an average secondary school student was estimated to be 0.036 μg/kg bw/day. The main dietary source of antimony was cereal and cereal products which contributed to 26.9% of the total exposure. Dietary exposures to antimony from different food groups are shown in Table 3.
Table 3: Dietary Exposure to Antimony for Average Secondary School Students
| Food groups | Dietary exposure to antimony in μg/kg bw/day (% contribution) |
|---|---|
| Cereal and cereal products | 0.010 (26.9%) |
| Vegetables | 0.007 (20.6%) |
| Fruits | 0.003 (8.5%) |
| Meat, poultry, egg and their products | 0.008 (21.8%) |
| Seafood | 0.005 (14.6%) |
| Dairy products | 0.003 (7.6%) |
| Total | 0.036 (100%) |
High consumers
31. Further analysis was undertaken to estimate the risk that high consumers might be exposed to. The 95th percentile exposure level of the secondary school students was used to represent the dietary exposure to antimony for a high consumer and was estimated to be 0.081 μg/kg bw/day (Table 4).
Table 4: Comparison between TDI Established by WHO and Dietary Exposure to Antimony for Average Secondary School Students and High Consumers
| WHO TDI (μg/kg bw/day) |
Exposure (μg/kg bw/day) (% of TDI) | |
|---|---|---|
| Average secondary school students | High consumers | |
| 6 | 0.036 (0.6%) | 0.081 (1.4%) |
RISK CHARACTERISATION
Dietary exposure
32. The dietary exposure to antimony for an average secondary school student was estimated to be 0.036 μg/kg bw/day whilst that for the high consumer was 0.081μg/kg bw/day. These exposures amounted to 0.6% and 1.4% of the TDI respectively. Exposures to antimony for both an average secondary school student and the high consumer fell well below the TDI established by WHO.
33. The dietary exposure estimates therefore suggested the average and high consumers of secondary school students were unlikely to experience major toxicological effects of antimony.
Effects of non-detected values
34. In this study, a value of 1/2-LOD was assigned to all analytical values below LOD. However, the true value of antimony in the food sample could be anywhere between zero and the LOD. To address this issue of uncertainty, antimony concentration in each food sample was also estimated using an upper bound and lower bound estimates. The upper bound was calculated by setting analytical values below LOD to the LOD while the lower bound was calculated by setting analytical values below LOD to zero.
35. Using these upper and lower bound estimates, the dietary exposure to antimony was calculated. The antimony exposure of an average secondary school student would be anywhere between 0.028 (lower bound estimate) and 0.044 (upper bound estimate) μg/kg bw/day while that of high consumers could be anywhere between 0.064 (lower bound estimate) and 0.096 (upper bound estimate) μg/kg bw/day. These exposures amount to 0.5 – 0.7% of TDI for an average student and 1.1 – 1.6% for the high consumer respectively.
Major dietary sources of antimony
36. In this study, the main dietary source of antimony was "cereal and cereal products" which contributed to 26.9% of the total exposure, and rice is a particularly significant source. Though rice had a relatively low level of antimony (average: 1 μg/kg), its relatively large amount of consumption made it also the largest contributor to total dietary exposure to antimony, i.e. 16.4%.
37. Following "cereal and cereal products", the food group "meat, poultry, egg and their products" ranked second in terms of contribution to dietary exposure to antimony and this was followed by "vegetables" (21.8% and 20.6% of dietary antimony exposures respectively).
38. Among different food groups, "dairy products" have the highest median concentration of antimony whereas that for the others fell below the LOD. However, its relatively low consumption pattern made it the least contributor to total dietary exposure to antimony, i.e. 7.6%. On the other hand, sausage was found to contain the highest level of antimony (average: 9 μg/kg) in this study. However, its low consumption pattern made it only accountable for 8.2% of total dietary exposure to antimony.
International comparison
39. Studies on dietary exposure to antimony conducted during 1990-2000s in overseas were reviewed and summarised in Table 5.
40. It can be seen that dietary exposure to antimony estimated in our study is in the same order of magnitude when compared to exposure estimates obtained from overseas studies. This comparison gives the reader a rough idea of the local situation relative to the international picture, but it should be noted that direct comparison of data between different studies has to be done with caution due to the differences in time when the studies were carried out, research methodology, food group categorisation, methods of collection of consumption data, methods of antimony analysis and methods of treating results below the detection limits.
Table 5. A Comparison of Average Daily Exposure to Antimony
| Places | Average Daily Dietary Exposure (μg/kg bw/day) |
|---|---|
| Australia 1 | <0.01-0.08 |
| France 3 | 0.017* |
| Hong Kong | 0.036† |
| UK 2 | 0.05† |
* The French total diet study reported that the dietary exposure of antimony of the average French population was 1 μg/day (i.e. about 0.017 μg/kg bw/day for a 60-kg adult).
† The exposure data in Hong Kong is extracted from our current study.
‡ The UK total diet study reported that the dietary exposure of antimony of the average UK population was 3 μg/day (i.e. about 0.05 μg/kg bw/day for a 60-kg adult).
Other sources of antimony exposure
41. Apart from food, the general population may also expose to antimony in air, drinking water or other beverages. In Hong Kong , data on the level of antimony in air is limited. Overseas study has been reported that the antimony exposure from air for a person living in the urban areas is estimated to be 0.06 -0.46 μg/day8 . On the other hand, the Water Supplies Department reported that the level of antimony in drinking water for the period April 2004 to March 2005 fell below 1 μg/L, which is well below the WHO guideline (1993) of 20 μg/L10. Assuming that a 60-kg adult consumed 2 L of drinking water per day, subsequent exposure to antimony was estimated to be below 0.033 μg/kg bw/day (i.e. < 0.6% of the TDI). Taking exposures from food and drinking water into account, oral exposure to antimony was still far below the TDI of 6 μg/kg bw/day.
42. A recent overseas study found that trace amount of antimony (up to 0.7 μg/L) was detected in bottled water upon storage because antimony compounds may be used as catalysts during the production of polyethylene terephthalate (PET), a common plastic material for making bottles for storing beverages. We collected 16 different bottled beverages, including water, carbonated drinks, juice drinks, tea and coffee for the analysis of antimony. The test results are summarised in Table 6.
Table 6. Concentration of Antimony in Bottled Beverages
| Type of beverages | Number of samples | Median concentration (μg/L) |
|---|---|---|
| Water | 3 | 0.1 |
| Tea | 3 | 0.6 |
| Coffee | 3 | 0.7 |
| Juice drinks | 3 | 1.1 |
| Carbonated beverages | 4 | 2.4 |
43. Taking into account the above data on level of antimony in bottled beverages and the food consumption pattern of secondary school students and assuming that all the beverages (except water from the mains) consumed was from PET bottles , an average secondary school students may have an additional exposure of 0.013 μg/kg bw/day (i.e. 0.2% of the TDI). The resulting overall dietary exposure to antimony was still far below the TDI of 6 μg/kg bw/day.
LIMITATION OF THE EXPOSURE ASSESSMENT STUDY
44. The methodology for collecting food consumption data may influence the accuracy of the estimates on dietary exposure. In the Food Consumption Survey, the food consumption pattern of secondary school students was collected using a food frequency questionnaire. Although the questionnaire was very comprehensive, it was not possible to cover every single food item, some of which might be relevant to antimony exposure. Furthermore, only the data of consumption pattern for secondary school students is available.
45. Three samples for each food item were combined into one composite sample for laboratory analysis. Although about 300 samples were taken in this study, increasing the number of sample for each food item for laboratory analysis could provide a more precise estimate of the average antimony concentration for a particular food item. However, the number of samples taken have to be balanced with the required resources and number of food items to be included.
CONCLUSION AND RECOMMENDATION
46. The dietary exposures to antimony for average and high consumers of the secondary school students were 0.036 and 0.081 μg/kg bw/day respectively. Both levels fell well below the safety reference value (i.e. < 2% of the TDI) established by WHO. It could be concluded that both the average and high consumers of the secondary school students were unlikely to experience major toxicological effects of antimony.
47. The food group "cereal and cereal products", particularly rice, was identified as the main dietary source of antimony.
48. Food has been recognised as the major source of antimony exposure for the general population. Because of its ubiquitous nature in the environment, low levels of antimony in foods might be unavoidable. However, the food trade is advised to observe good agricultural and manufacturing practices to minimise antimony contaminations in food.
49. The public is also advised to maintain a balanced diet so as to avoid excessive exposure to metallic contaminants from a small range of food items.
REFERENCES
1 UK Ministry of Agriculture, Fisheries and Food (MAFF). 1994 Total Diet Study (Part 2) – Dietary intakes of metals and other elements. Joint Food Safety and Standards Group – Food surveillance information sheet No. 149. London : MAFF; May 1998. [cited 6 Feb 2006 ] Available from: URL: http://archive.food.gov.uk/maff/archive/food/infsheet/1998/no149/149tds.htm
2 Food Standards Australia New Zealand (FSANZ). The 20 th Australian Total Diet Survey – a total diet survey of pesticide residues and contaminants. Canberra : FSANZ; January 2003.
3 Leblanc Jean-Charles et al. Dietary estimates of 18 elements from the 1 st French total diet study. Food Additives and Contaminants 2005; 22(7): 624-641.
4 US Agency for Toxic Substances and Disease Registry. Toxfaqs: antimony. Atlanta : ATSDR; September 1995. Available from: URL: http://www.atsdr.cdc.gov/tfacts23.html
5 US Agency for Toxic Substances and Disease Registry. Toxicological profile for antimony. Atlanta : ATSDR; December 1992. Available from: URL: http://www.atsdr.cdc.gov/toxprofiles/tp23.html
6 Health Canada . Antimony – Guidelines for Canadian drinking water quality: supporting documentation. Ottawa : Canada ; May 1997 (edited August 1999).
7 IARC. Antimony trioxide and antimony trisulfide – summaries & evaluation. Lyon : IARC; 1989. [cited 26 Aug 2004 ] Available from: URL: http://www.inchem.org/documents/iarc/vol47/47-11.html
8 WHO. Antimony in drinking-water – background document for development WHO Guidelines for Drinking-water Quality. Geneva : WHO; 2003.
9 FEHD. Food Consumption Survey 2000. Hong Kong : FEHD; 2001.
10 Water Supplies Department. Drinking water quality for the period April 2005 - March 2006. Hong Kong : WSD; 2006. [cited 30 Jun 2006 ] Available from: URL: http://www.wsd.gov.hk/en/html/pdf/wq/drinking_b-e.pdf
11 Shotyk W, Krachler, M and Chen, B. Contamination of Canadian and European bottled waters with antimony from PET containers. Journal of Environmental Monitoring 2006; 8: 288-292.