Cultured meat is recently a topic attracting increasing interest. Cultured meat, also referred to as lab-grown meat, in vitro meat, etc, is meat produced from animal cell culture techniques. It is intended to be consumed as an alternative to conventional meat products.
Production of Cultured Meat
To produce cultured meat, cells are first collected from target animals and allowed to proliferate in a culture medium under a controlled environment. The cells may also be grown onto a supporting structure referred to as a scaffold which provides an anchor for cell attachment and growth to produce products resembling meat muscle or other animal parts targeted to be produced.
After the cells have formed a sufficient amount, the cellular materials can be harvested from the growth medium. If a scaffold is used, it will be separated from the cultured meat or left attached if the scaffold is edible. The harvested cultured meat is prepared into the final product.
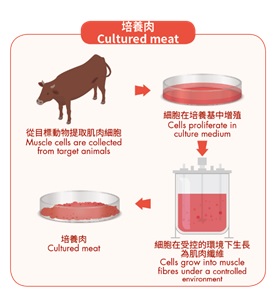
Production process of cultured meat, e.g. from muscle cells
Safety Assessment of Cultured Meat
The production of cultured meat involves animal cell culture technology that is relatively new to food production and significantly different from the production of conventional meat products. There is thus concern on the potential food safety risks to consumers.
According to section 54 of the Public Health and Municipal Services Ordinance (Cap. 132), all food available for sale in Hong Kong must be fit for human consumption. Before importing cultured meat into Hong Kong for sale, or manufacturing it locally for human consumption, the trade should first submit data on safety assessment of the cultured meat to the Centre for Food Safety for evaluation. If the trade has any enquiries concerning the safety assessment, please contact the Centre for Food Safety at the following e-mail address:
In the safety assessment of cultured meat, the information required generally involves information on production, data on composition, allergenicity, toxicological and nutritional aspects, etc. Aspects evaluated in the safety assessment of cultured meat generally include:
- Characterisation of the cultured meat
- Production process
- Compositional data
- Specifications
- Proposed uses and levels of consumption
- Absorption, distribution, metabolism and excretion (ADME)
- Nutritional information
- Toxicological information
- Allergenicity
- Analytical detection method
- Any safety assessment reports conducted by food safety authorities in other countries / places; and
- Any other relevant information to support the safety.
The Centre for Food Safety has prepared the “Technical Guidance Notes on the Safety Assessment of Cultured Meat” to provide technical guidance to the trade on the requirements regarding the safety assessment of cultured meat. Please click the following link to download the guidance notes:
Technical Guidance Notes on the Safety Assessment of Cultured Meat
Advisory Panel on the Safety Assessment of Cultured Meat
The Centre for Food Safety has set up an Advisory Panel on the Safety Assessment of Cultured Meat to provide advice to the Director of Food and Environmental Hygiene on the safety assessment of cultured meat. For details of membership and terms of reference of the Advisory Panel, please click here.