Genetic modification in food production and processing
Ever since humans have grown plants and raised animals for food, we have selected plants and animals with beneficial traits for further breeding. Modern biotechnology now makes it possible to alter genetic material to create novel traits in plants, animals, bacteria and fungi. This technology has so far primarily been used in crops to increase insect resistance and herbicide tolerance, and less familiar to the general public, in microorganisms to produce food processing enzymes.
Enzymes for food processing
Enzymes are protein molecules that are present in all living organisms and speed up biochemical reactions necessary to support life. They are present in fresh and processed food that we consumed every day.
Enzymes are used unknowingly in food processing and production, e.g. dough making, for centuries. In the past, enzymes were isolated mainly from plant and animal sources, and thus a relatively limited number of enzymes were available to food processers at a high cost. Today, microorganisms such as bacteria and fungi are exploited and some of which are genetically modified for the commercial production of a diversity of enzymes that are tailored to specific food processing conditions. A well-known example is the production of calf chymosin for cheese making with genetically modified microorganisms (GMMs).
Chymosin
Chymosin, which causes milk to curdle, is an important enzyme for cheese production. Traditionally, chymosin was extracted from calves’ stomachs. Because of the rising cheese production and a decrease in the number of calves being slaughtered, it was difficult to secure sufficient amount of raw material at stable prices for the production of chymosin and resulted in the search for alternate clotting agents.
The application of modern biotechnology made it possible to produce chymosin from microorganisms and alleviates the dependence on the supply of calf stomachs. In practice, the gene encoding chymosin in calves is isolated and introduced into microorganisms such as bacteria, fungi and yeasts (see Figure). These GMMs are cultured and allowed to produce chymosin under controlled conditions. Chymosin thus produced is extracted, purified and used to curdle milk in cheese production. Now about 80% of the total cheese production in the United States is made with chymosin produced by GMMs.
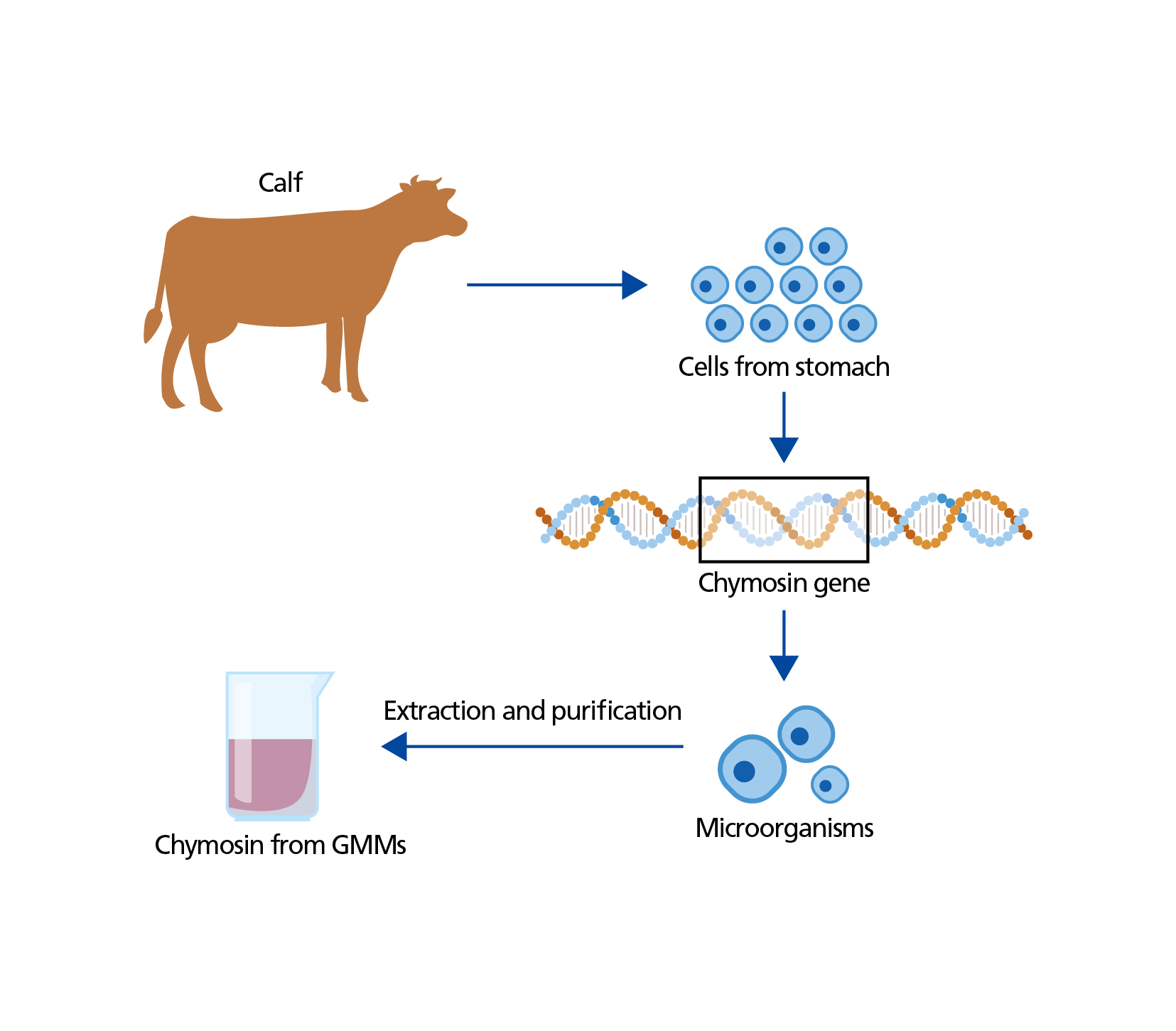
Figure: Production of chymosin with GMMs. Chymosin gene isolated from calf stomach is transferred to appropriate producer organisms such as bacteria, fungi or yeasts. These GMMs are cultivated in controlled conditions, in which they produce the enzyme. Chymosin is separated and is cleaned of possible impurities including cell debris from the GMMs.
Safety of food processing enzymes produced by GMMs
In general, food enzymes produced industrially by fermentation of microorganisms, including those produced from genetically modified microbial sources, are assessed for safety before marketing.
In principle, the same safety considerations apply to enzymes derived from GMMs and non-GMMs. The safety of enzymes is evaluated by considering 1) the possible hazard of the production organism; 2) properties of the enzyme; and 3) the toxicity of the enzyme preparation intended for commercial use.
Safety of the production microorganism
The key component of evaluating food enzyme safety from microbial sources is the safety assessment of the production strain. Microorganisms used for enzyme production should be well-characterised and not produce any pathogens or toxins. Their history of safe use in food processing is also an important factor to be taken into account.
Enzyme properties
The chemical composition and the properties of the enzymes are considered when evaluating the safety of food enzymes. For enzymes produced with GMMs, further analysis on the characteristics of the introduced DNA will be conducted to ensure the enzymes have no significant homology to known protein allergens or toxins.
Safety of the enzyme preparation
Moreover, the fermentation process for enzyme production should be conducted under controlled conditions to prevent contamination with microorganisms that could be the source of toxic and other undesirable substances. In addition, culture media used to grow microorganisms should consist of components that leave no residues harmful to health in the finished product. Finally, the likely amount of food enzyme that would be consumed is estimated to ensure there will be no adverse effect to human health when the enzyme is used at the level suggested by the manufacturer.
Standard and regulation of food processing enzymes
Enzymes produced by GMMs are usually extracted, purified and used as processing aids in the food industry. Purified enzymes are cell free entities and do not contain any other macromolecules such as DNA. In the European Union, the United States, Australia and New Zealand where there are specific legislations on GM food, food containing enzymes derived from GMMs do not fall under the scope of the legislations if the GMMs are not present in the final product.
Although Codex Alimentarius does not have standards for processing aids or enzymes and different places may regulate the use of enzymes differently, in Hong Kong, according to Section 54 of the Public Health and Municipal Services Ordinance (Cap 132), all food intended for human consumption for sale in Hong Kong, whether imported or locally produced, must be fit for human consumption. Traders may make reference to internationally recognised specifications for enzymes, including those produced from genetically modified microbial sources, established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA)* for the identity and purity of specific food processing enzymes.
* The Joint FAO/WHO Expert Committee on Food Additives (JECFA) is an international scientific expert committee administered jointly by the Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO).