Stop sale and consumption of a plasticiser-tainted dietary supplement imported from US
| Issue Date | 14.7.2011 |
|---|---|
| Source of Information | Centre for Food Safety (CFS) |
| Food Product | Dietary supplement |
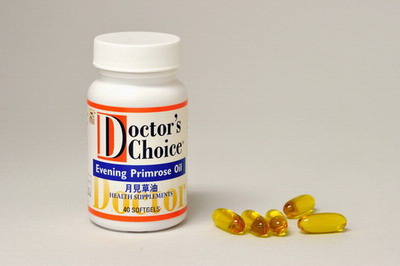
| Product Name and Description | Brand name and food name: Doctor's Choice – Evening Primrose Oil
|
| Reason For Issuing Alert | The concerned product was found to contain plasticisers, di(2-ethylhexyl) phthalate (DEHP) and di-butyl phthalate (DBP). Plasticisers have been found to cause damages to liver, kidney, reproduction and development when given to animals in large doses. |
| Action Taken by the Centre for Food Safety | The CFS has instructed the sole importer, Vita Green Health Products Co, Ltd, to immediately stop the sale and initiate recall of the relevant batch of the product. |
| Advice to the Trade | Stop selling the affected product. |
| Advice to Consumers | Stop consuming the affected product. |
| Further Information |
CFS Website: Plasticisers in food For enquiries about the product and recall arrangements, please call the consumer hotline set up by the importer during office hours (Tel: 2901 6000). |
Centre for Food Safety
Food and Environmental Hygiene Department
2011-7-14