
I. The Regulation
Q 1 Can a follow-up formula be specified for persons at any age between 4 months or above and under 9 months?
A 1 No. Under the Amendment Regulation, follow-up formula must not be marked or labelled to the effect that the formula is suitable for consumption by persons of any age under 6 months.
Q 2 If a prepackaged food is specified for persons at any age between 1 year or above and under 5 years, how will it be regulated?
A 2 As such product is claimed to be suitable for consumption of persons aged both at or above and under 36 months, it will be regulated as both prepackaged food for infants and young children (provided that it is not an infant formula or follow-up formula as defined) and general prepackaged food. That means it should observe the relevant nutrition labelling requirements in the Amendment Regulation and the existing Nutrition Labelling Scheme. Nutrient contents needed to be declared for prepackaged food for infants and young children in the Amendment Regulation and for general prepackaged food in the Nutrition Labelling Scheme are as follows:
| Nutrient contents needed to be declared for prepackaged food for infants and young children in the Amendment Regulation | Nutrient contents needed to be declared for general prepackaged food in the Nutrition Labelling Scheme |
|---|---|
| Energy | Energy |
| Protein | Protein |
| Total fat | Total Fat |
| Available carbohydrates | Saturated fatty acids |
| Sodium | Trans fatty acids |
| Vitamin A (if added) | Available carbohydrates |
| Vitamin D (if added) | Sugars |
| Sodium | |
| Nutrients involved in nutrition claim (s) |
If such product is a follow-up formula as defined, it should observe relevant nutrition labelling requirements in the Amendment Regulation and the existing Nutrition Labelling Scheme.
Q 3 Is there any requirement on the protein content of infant formula which based on sources other than cows milk protein and soy protein isolate (e.g. goats milk protein)?
A 3 The Amendment Regulation makes reference to the Standard for Infant Formula and Formulas for Special Medical Purposes Intended for Infants (CODEX STAN 72-1981) and includes nutritional composition requirements of infant formula, including the protein content for infant formula based on cows milk protein and soy protein isolates. As there is no specific requirement in CODEX STAN 72-1981 on the protein content of the infant formula based on sources other than cows milk protein and soy protein isolate and no international consensus on such requirement, protein content of the infant formula based on sources other than cows milk protein and soy protein isolate is not covered by the nutritional composition requirement in the Amendment Regulation. Nevertheless, it is recommended that the protein content of such formula should fulfill the requirement set out by the jurisdiction or country where the product is originated.
Q 4 Some milk products intended for consumption by adults would be labelled as "Children under one year of age should not be fed on this milk except under medical advice". Will this type of product be covered by the Amendment Regulation?
A 4 This type of product would generally not be covered by the Amendment Regulation provided that it does not have any specific indication that it is intended for persons under the age of 36 months. If it has any indication (e.g. product description or instruction for use) that it is intended for consumption by persons under the age of 36 months, it would be regulated by the Amendment Regulation and should observe the relevant nutritional composition and/or nutrition labelling requirements.
II. Product Definition
Q 5 What are the examples of "formula for special medical purposes for infants and young children"?
A 5 Formula for infants and young children with metabolic diseases (e.g. maple syrup urine disease or phenylketonuria) and for specific groups of infants and young children such as premature infants or babies with lactose intolerance, are considered as formula for special medical purposes for infants and young children. Formula for special medical purposes for infants and young children should be marked or labelled as stated in Annex I in the "Technical Guidance Notes on Nutritional Composition and Nutrition Labelling of Infant Formula, Follow-up Formula and Prepackaged Food for Infants and Young Children" in order to be exempted from the nutritional composition and nutrition labelling requirements in the Amendment Regulation. It should be noted that the presentation of the product as formula for special medical purposes for infants and young children needs to observe the relevant provisions in the Amendment Regulation and where applicable, other laws of Hong Kong including the Undesirable Medical Advertisements Ordinance (Cap. 231).
Q 6 What kinds of product would be considered as infant formula in the Amendment Regulation?
A 6 Formula product which is intended for consumption as a substitute for human breast milk that is specially manufactured to satisfy, by itself, the nutritional requirements of persons of any age up to and including 12 months until the introduction of appropriate complementary feeding, or marked or labelled as "infant formula" ( 嬰兒配方產品 ) or with any other words of similar meaning would be considered as infant formula. For instance, formula product which is marked or labelled as "infant milk", "new-born formula", "new-born milk", " 初生嬰兒配方奶粉 " or " 嬰兒配方奶粉 " would be considered as infant formula and should observe nutritional composition and nutrition labelling requirements for infant formula in the Amendment Regulation.
Q 7 What kinds of product would be considered as follow-up formula in the Amendment Regulation?
A 7 Formula product which is represented as a replacement for human breast milk or infant formula (including its replacement or subsequent replacement), and intended for consumption as a liquid element in a progressively diversified diet by persons of any age from 6 months to under 36 months would be considered as follow-up formula. Formula product that marked or labelled as "follow-up formula" ( 較大嬰兒及幼兒配方產品 ) or with any other words of similar meaning would also be considered as follow-up formula. For instance, formula product which is marked or labelled as "follow-on formula", "follow-on milk", "growing-up formula", "growing-up milk", "toddler formula", "toddler milk", " 較大嬰兒配方奶粉 ", " 幼兒助長配方奶粉 " or " 幼兒成長配方奶粉 " would be considered as follow-up formula and should observe nutrition labelling requirements for follow-up formula in the Amendment Regulation.
Products like fruit juice, vegetable juice, instant powdered drink or fresh milk will generally not be considered as "follow-up formula". However, it should be noted that prepackaged food which is intended for consumption by persons of any age under 36 months (excluding infant formula and follow-up formula) would be considered as prepackaged food for infants and young children and should observe relevant nutrition labelling requirements in the Amendment Regulation.
III. Nutrition Labelling
Q 8 How can I know that vitamin A or vitamin D is added to a prepackaged food for infants and young children ?
A 8 Vitamin A (or its other forms such as retinol and β-carotene) or vitamin D (or its other forms such as cholecalciferol and ergocalciferol) should be listed in the ingredient list if they are added.
Q 9 Certain nutrients have different definitions in infant formula/follow-up formula and general prepackaged food. If a product is considered as follow-up formula and general prepackaged food, and makes claims on such nutrients, how should the content of such nutrients be labelled?
A 9 According to the Amendment Regulation, folic acid, niacin, vitamin A , vitamin C, vitamin E and vitamin K have different definitions in infant formula/follow-up formula and general prepackaged food. If a product is considered as follow-up formula and general prepackaged food, and makes claims on such nutrients, the contents of such nutrients should be labelled according to the Amendment Regulation and the existing Nutrition Labelling Scheme. To avoid doubt, it is recommended to clearly label the content of such nutrients according to their definition in follow-up formula and general prepackaged food. The example below shows the recommended way to label such nutrients in aforesaid scenario.
| Nutrition Information | |
|---|---|
| Per 100g | |
| ---- | ---- |
| ---- | ---- |
| Folic acid (N-pteroyl-L-glutamic acid) Folic acid |
xx µg xx µg DFE |
| Niacin Nicotinamide |
xx µg xx µg |
| Vitamin A (all-trans retinol) Vitamin A (retinol and beta-carotene) |
xx µg RE xx µg RE |
| Vitamin C (ascorbic acid) Vitamin C |
xx mg xx mg |
| Vitamin E (alpha-tocopherol compounds) Vitamin E |
xx mg α-TE xx mg α-TE |
| Vitamin K Vitamin K1 |
xx µg xx µg |
| ---- | ---- |
IV. Exemption
Q 10 What other words are deemed to have similar meaning with "formula for special medical purposes" ( 特殊醫用配方產品 ) and "USE UNDER MEDICAL SUPERVISION" ( 在醫生指示下使用 ) in the labelling requirements for exemption of formula for special medical purposes for infants and young children (FSMP) from the nutritional composition and nutrition labelling requirements?
A 10 The table below (which is not exhaustive) lists out some words that are considered to be of similar meaning with "formula for special medical purposes" ( 特殊醫用配方產品 ) and "USE UNDER MEDICAL SUPERVISION" ( 在醫生指示下使用 ):
| Words with similar meaning | |
|---|---|
| formula for special medical purposes ( 特殊醫用配方產品 ) |
- Formula for special medical uses - Special medical purpose formula - Special medical use formula - 特殊醫療用途配方產品 |
| USE UNDER MEDICAL SUPERVISION ( 在醫生指示下使用 ) |
- USE UNDER MEDICAL ADVICE - USE UNDER SUPERVISION OF A MEDICAL PROFESSIONAL - USE AS DIRECTED BY A MEDICAL PROFESSIONAL - 在醫生監督下使用 - 在醫護人員指導下使用 |
V. Testing and Analysis
Q 11 Do the tolerance limits cover the measurement uncertainty of test methods?
A 11 Measurement uncertainty is a quantitative indicator of the analytical variability of a test result and has not been taken into consideration when corresponding tolerance limits were set. Since measurement uncertainty and tolerance limit are independent of each other, the measurement uncertainty will be dealt with separately in the event of compliance checking.
Q 12 Which organizations provide proficiency test for macronutrients?
A 12 Food Analysis Performance Assessment Scheme (FAPAS) of the Food and Environment Research Agency, United Kingdom organizes a number of proficiency tests (PT) on macronutrients, including moisture, ash, total fat, nitrogen, etc. in infant formula and milk powder. Similarly, the Laboratory of Government Chemist, United Kingdom organizes PT for most macronutrients in ready-to-eat and cereal products.
(Please be reminded that PT providers are not limited to the organizations mentioned in the "Method Guidance Notes on Nutritional Composition and Nutrition Labelling of Infant Formula, Follow-up Formula and Prepackaged Food for Infants and Young Children". Laboratory can search for other PT scheme that offer relevant food matrix and test parameter. CFS recommends the selection of PT providers that are accredited to ISO/IEC 17043 standard as a first choice.)
Q 13 Which organizations provide proficiency test for micronutrients, including vitamins, in formula products?
A 13 "Bureau Interprofessionnel des Etudes Analytiques" (BIPEA – International Bureau for Analytical Studies) organizes a comprehensive PT programme (dietary products and nutritional labeling) on micronutrients in infant milk / baby milk. The main parameters includes fluoride, iodide, lauric acid, Ca, Cu, Fe, K, Mg, Mn, Na, Se, P, Zn, vitamins (A, B1, B2, B5, B6, B9, B12, C, D, E, H, K, PP), choline, carnitine, inositol, etc. Besides, FAPAS organizes PT on vitamin A, D3 and elements in milk powder.
(Please be reminded that PT providers are not limited to the organizations mentioned in the "Method Guidance Notes on Nutritional Composition and Nutrition Labelling of Infant Formula, Follow-up Formula and Prepackaged Food for Infants and Young Children". Laboratory can search for other PT scheme that offer relevant food matrix and test parameter. CFS recommends the selection of PT providers that are accredited to ISO/IEC 17043 standard as a first choice.)
Q 14 How to interpret proficiency test findings?
A 14 There is a number of different ways to present the PT findings. One of the common ways is z-score. If the z-score value is between -2 and +2, it is classified as ‘satisfactory'. When the z-score value is between -2 and -3 or +2 and +3, it is normally classified as ‘questionable'. For z-score value is less than -3 or greater than +3, the result is said to be ‘unsatisfactory' and the laboratory needs to investigate the source of unsatisfactory result.
Q 15 Do I need to determine available carbohydrates before calculate energy content of an infant formula?
A 15 For prepackaged food, energy is obtained by the summation of the energy contributed by available carbohydrates, protein, total fat, ethanol, and organic acids, multiplied by corresponding conversion factors. However, the Codex's "Standard for infant formula and formulas for special medical purposed intended for infants, CODEX STAN 72-1981 (Amended 2011)" ("Codex Stan 72") has set minimum and maximum level for ‘total carbohydrates' in infant formula only and there is no specific requirement set for dietary fibre in this standard. Therefore, ‘total carbohydrates' is used for energy calculation and ‘Energy' in infant formula is calculated by the following formula:
(weight in grams [4 x total carbohydrates + 4 x protein + 9 x total fat] kcal in 100 mL of infant formula prepared ready for consumption)
For follow-up formula and prepackaged food for infants and young children, ‘available carbohydrates' is used for energy calculation. Please refer to the "Method Guidance Notes on Nutrition Labelling and Nutrition Claims" for the energy calculation formula.
Q 16 Which nitrogen to protein conversion factor should be used for infant formula based on soy protein or goat's milk protein ?
A 16 According to Codex Stan 72, nitrogen to protein conversion factor should be based on 6.25 unless a scientific justification for the use of a different conversion factor for a particular product. Besides, most countries that regulate infant formula used 6.25 as nitrogen to protein conversion factor. Thus, CFS uses 6.25 as nitrogen to protein conversion factor too. Please note that Australia and New Zealand use 6.38 as conversion factor for milk proteins and their partial protein hydrolysates.
Q 17 Regarding the additional requirements on lauric, myristic and erucic acid and trans fatty acids, can total fat instead of total fatty acids be used for calculation?
A 17 In the Amendment Regulation, it is stated clearly that the requirements were based on total fatty acids. Using total fat for calculation would lead to underestimation since total fat also contains phospholipids, wax ester, sterols and other minor amount of non-fatty material.
Q 18 What is polyunsaturated fat?
A 18 Polyunsaturated fat refers to fatty acids with cis-cis methylene double bonds and commonly the sum of 14 polyunsaturated fatty acids including C18:2(9,12-cis), C18:3(6,9,12-cis), C18:3(9,12,15-cis), C20:2(11,14-cis), C20:3(8,11,14-cis), C20:3(11,14,17-cis), C20:4(5,8,11,14-cis), C20:5(5,8,11,14,17-cis), C22:2(13,16-cis), C22:3(13,16,19-cis), C22:4(7,10,13,16-cis), C22:5(4,7,10,13,16-cis), C22:5(7,10,13,16,19-cis) and C22:6(4,7,10,13,16,19-cis).
Q 19 Does beta-carotene count as vitamin A in infant and follow-up formula?
A 19 With reference to the Codex Stan 72 and 156, the Amendment Regulation recognizes only all trans-retinol as vitamin A. Therefore, the method used for quantification should be able to separate the all trans-retinol from other stereoisomers. Please note that beta-carotene counts as vitamin A for prepackaged food including prepackaged food for infants and young children.
Q 20 Is there any difference for definition of vitamin E in infant formula, follow-up formula and prepackaged food for infants and young children?
A 20 Only d-alpha-tocopherol is defined as vitamin E in infant formula while both d- and l- isomer of alpha-tocopherol are counted as vitamin E in follow-up formula. For prepackaged food for infants and young children, please refer to FAQ 27 of the "Method Guidance Notes on Nutrition Labelling and Nutrition Claims" for details.
Q 21 How can I distinguish vitamin E obtained from natural source or synthetic source?
A 21 Vitamin E obtained from synthetic source is a racemic mixture. In order to distinguish them, chiral separation of alpha-tocopherol isomers is necessary.
Q 22 How to calculate vitamin E in terms of IU if a follow-up formula contains vitamin E from both natural source and synthetic source?
A 22 In such case, both d- and l- form of alpha-tocopherol should be determined. Since synthetic sources of vitamin E consists of racemic mixtures, i.e. d- and l- form in 1:1 ratio, the content of synthetic form can be found. Subsequently, the content of natural form can be calculated.
Q 23 Are there suitable methods for separating erythorbic acid, L-dehydroascorbic acid and ascorbic acid in formula products?
A 23 The D-isomer (erythorbic acid) of ascorbic acid, which is used as an antioxidant food additive, does not show vitamin C activity. Hence, it is a good suggestion to determine these compounds simultaneously. At present, standard method cannot be found to separate them simultaneously. However, Doner and Hicks (Doner L.W., Hicks K.B. (1981) High-Performance Liquid Chromatographic Separation of Ascorbic Acid, Erythorbic Acid, Dehydroascorbic Acid, Dehydroerythorbic Acid, Diketogulonic Acid, and Diketogluconic Acid Analytical Biochemistry 115:225-230) of US Department of Agriculture and Kall and Andersen (Kall M.A, Andersen C. (1999) Improved method for simultaneous determination of ascorbic acid and dehydroascorbic acid, isoascorbic acid and dehydroisoascorbic acid in food and biological samples Journal of Chromatography B 730:101-111) of Danish Veterinary and Food Administration published their developed methods to quantify these compounds in foods and are suitable references.
Q 24 Is AOAC 985.34 suitable for testing niacin in follow-up formula?
A 24 AOAC 985.34 determines nicotinic acid and nicotinamide by microbiological assay and cannot provide the content of nicotinic acid and nicotinamide separately. Hence, the quantitative results of niacin may be overestimated.
Q 25 N-pteroyl-L-glutamic acid is another name for folic acid. Does it need to be defined?
A 25 The ‘folic acid' referred in the Amendment Regulation is free folic acid and does not include food bounded folate. Thus, digestion or hydrolysis step before extraction and/or quantification should be avoided. Otherwise, overestimation may occur.
Q 26 How to calculate vitamin E to polyunsaturated fatty acids ratio?
A 26 The denominator of the ratio depends on the composition of polyunsaturated fatty acids (PUFA). The general formula for calculating the minimum ratio is given below:
Minimum α-TE/g PUFA (mg/g PUFA) = {0.5 x [C18:2(9,12-cis) + C20:2(11,14-cis) + C22:2(13,16-cis)] + 0.75 x [C18:3(6,9,12-cis) + C18:3(9,12,15-cis) + C20:3(8,11,14-cis) + C20:3(11,14,17-cis) + C22:3(13,16,19-cis)] + 1.0 x [C20:4(5,8,11,14-cis) + C22:4(7,10,13,16-cis) ] + 1.25 x [C20:5(5,8,11,14,17-cis) + C22:5(4,7,10,13,16-cis) + C22:5(7,10,13,16,19-cis) ] + 1.5 x [C22:6(4,7,10,13,16,19-cis)]} / {C18:2(9,12-cis) + C20:2(11,14-cis) + C22:2(13,16-cis) + C18:3(9,12,15-cis) + C18:3(9,12,15-cis) + C20:3(8,11,14-cis) + C20:3(11,14,17-cis) + C22:3(13,16,19-cis) + C20:4(5,8,11,14-cis) + C22:4(7,10,13,16-cis) + C20:5(5,8,11,14,17-cis) + C22:5(4,7,10,13,16-cis) + C22:5(7,10,13,16,19-cis) + C22:6(4,7,10,13,16,9-cis)} where [Cx:y(n1,n2…-cis)] refers to the concentration of individual PUFAs with the same concentration unit.
Using the above equation, for an infant formula sample containing PUFA at the level of 7.92 g/100g, of which the concentrations of C18:2(9,12-cis), C18:3(9,12,15-cis) and C22:6(4,7,10,13,16,19-cis) are 7.54g/10 g, 0.30g/100g and 0.08g/100g respectively, the minimum content of vitamin E is calculated to be 0.52 mg per g PUFA in sample.
Q 27 Is AOAC 954.05 or 905.03 suitable for testing fluoride in infant formula?
A 27 AOAC 954.05 and 905.03 are qualitative methods only and cannot be used for quantitative analysis of fluoride in infant formula. In order to determine fluoride in food quantitatively, AOAC 944.08 may be considered. Other fully validated methods for quantitative determination of fluoride in infant formula can also be used.
Q 28 How will the Administration calculate the fluoride content of infant formula in a form that is reconstituted or served?
A 28 Ultrapure water will be used when testing the fluoride content of infant formula. When calculating the fluoride content of infant formula in a form that is reconstituted or served, the Administration will take the instruction for use (including the type and amount of water to be used for reconstitution as recommended by the manufacturer) and the fluoride content of the drinking water in Hong Kong into account. If the product does not have any special recommendation on the type of water to be used or only shows that general drinking water can be used for reconstitution on its instruction for use, fluoride content in water (the average fluoride contents of drinking water in Hong Kong were 0.48-0.49 mg/L in 2009-2013) of 0.5mg/L will be used for calculation.
For example, 13g infant formula powder required 90ml water to make up 100ml infant formula with energy value of 65kcal. If the tested fluoride content of the infant formula powder using ultrapure water is 150µg/100g, according to the following equation,
Fluoride content of reconstituted infant formula (µg /100kcal) =
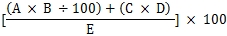
A= fluoride content of infant formula powder (µg/100g)
B= infant formula powder used during reconstitution of 100mL infant formula (g)
C= fluoride content of water (mg/L)
D= water used during reconstitution of 100mL infant formula (mL)
E= energy value in 100mL reconstituted infant formula (kcal)
the fluoride content of reconstituted infant formula will be 99.2µg/100kcal, which does not exceed 100µg/100kcal. As a result, the infant formula is not required to be labelled with a statement associated with dental fluorosis.